|
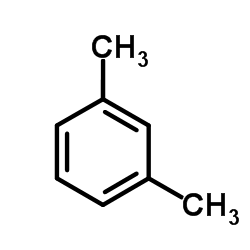
~40% |
|
~24%
详细
|
|
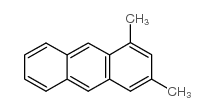
~60% |
|
~0% |
|
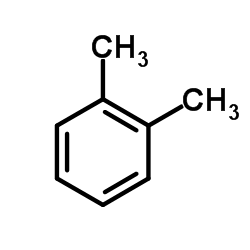
~49% |
|
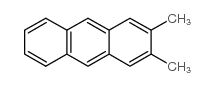
~35% |
|
~29% |
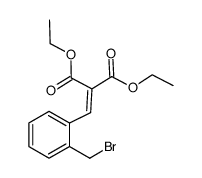
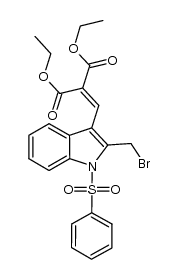

![ethyl 3-oxo-10-(phenylsulfonyl)-3,10-dihydro-1H-oxepino[3,4-b]indole-4-carboxylate结构式](https://image.chemsrc.com/caspic/117/1072096-82-2.png)

![5-(benzenesulfonyl)benzo[b]carbazole结构式](https://image.chemsrc.com/caspic/489/89241-42-9.png)
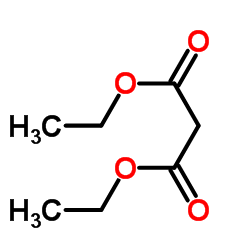
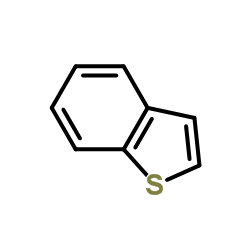
![苯并[b]萘并[2,3-d]噻吩结构式](https://image.chemsrc.com/caspic/110/243-46-9.png)