|
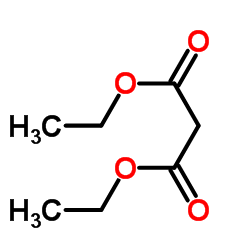
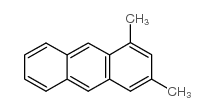
~40% |
|
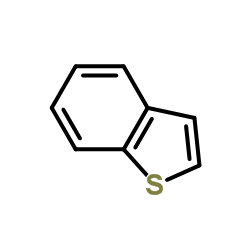
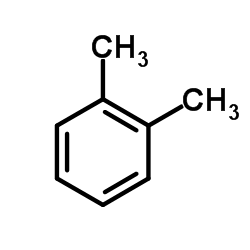
~24%
Detail
|
|
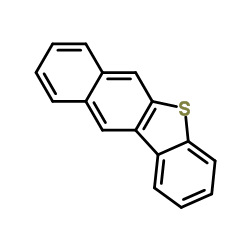
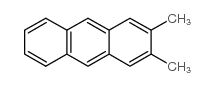
~60% |
|
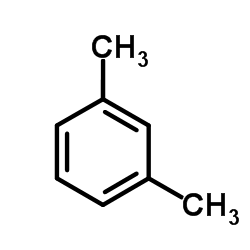
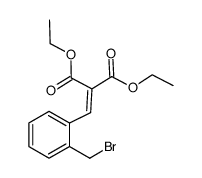
~0% |
|
~49% |
|
~35% |
|
~29% |
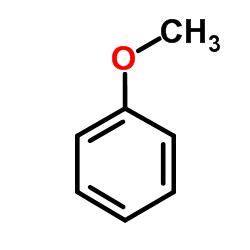
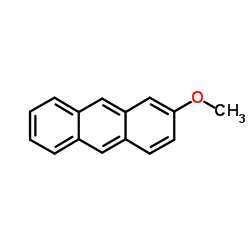
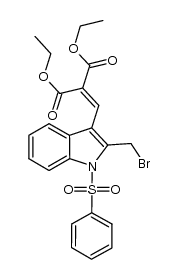
![ethyl 3-oxo-10-(phenylsulfonyl)-3,10-dihydro-1H-oxepino[3,4-b]indole-4-carboxylate Structure](https://image.chemsrc.com/caspic/117/1072096-82-2.png)

![5-(benzenesulfonyl)benzo[b]carbazole Structure](https://image.chemsrc.com/caspic/489/89241-42-9.png)