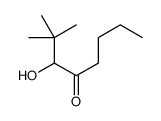
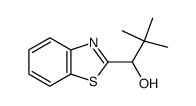
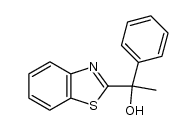
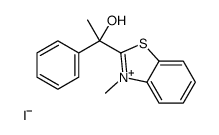
|
~74% |
|
~76% |
|
~81% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~83% |
|
~% |
|
~% |
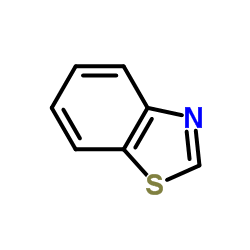

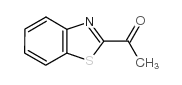
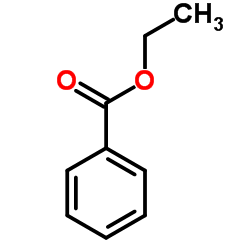
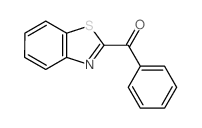
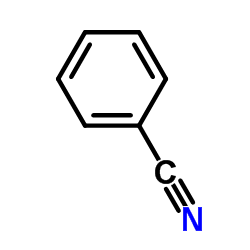
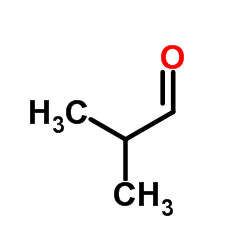
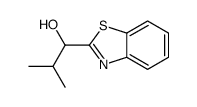
![1-(2-butyl-3-methyl-2,3-dihydrobenzo[d]thiazol-2-yl)-2,2-dimethylpropan-1-ol结构式](https://image.chemsrc.com/caspic/338/120822-15-3.png)