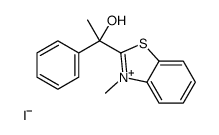
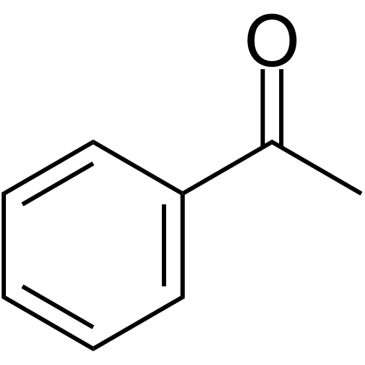
|
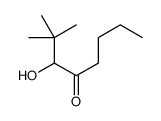
~74% |
|
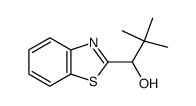
~76% |
|
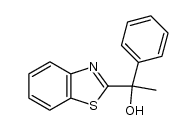
~81% |
|
~% |
|
~% |
|
~% |
|
~% |
|
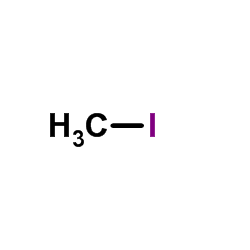
~83% |
|
~% |
|
~% |
![Benzo[d]thiazole Structure](https://image.chemsrc.com/caspic/318/95-16-9.png)

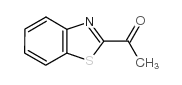
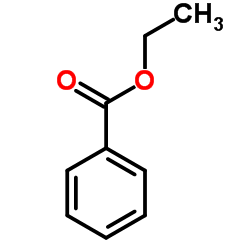
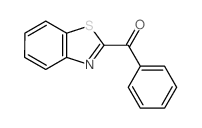
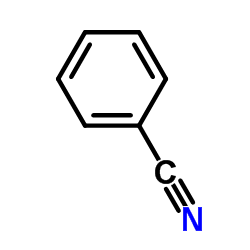
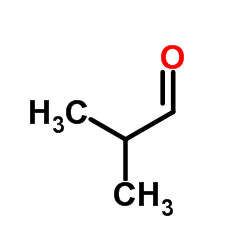
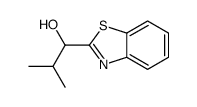
![1-(2-butyl-3-methyl-2,3-dihydrobenzo[d]thiazol-2-yl)-2,2-dimethylpropan-1-ol Structure](https://image.chemsrc.com/caspic/338/120822-15-3.png)