Tamoxifen-d3
Modify Date: 2024-01-16 17:36:05
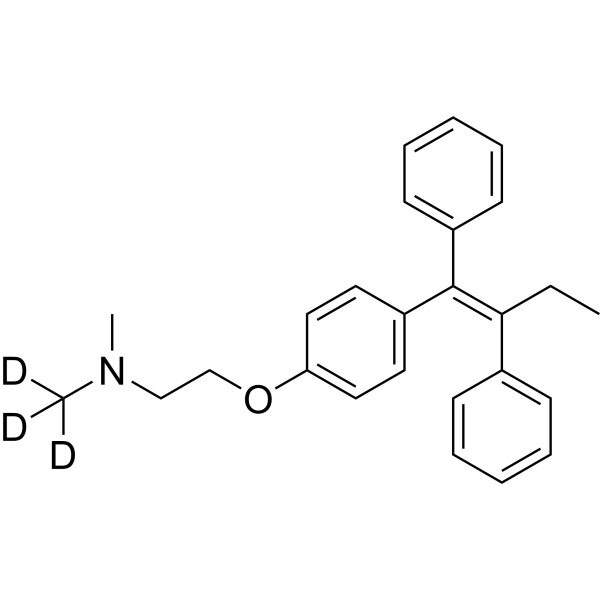
Tamoxifen-d3 structure
|
Common Name | Tamoxifen-d3 | ||
|---|---|---|---|---|
| CAS Number | 508201-30-7 | Molecular Weight | 374.53 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C26H26D3NO | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Tamoxifen-d3Tamoxifen-d3 is the deuterium labeled Tamoxifen[1]. Tamoxifen (ICI 47699) is an orally active, selective estrogen receptor modulator (SERM) which blocks estrogen action in breast cells and can activate estrogen activity in other cells, such as bone, liver, and uterine cells[2][3][4]. Tamoxifen is a potent Hsp90 activator and enhances the Hsp90 molecular chaperone ATPase activity. Tamoxifen also potent inhibits infectious EBOV Zaire and Marburg (MARV) with IC50 of 0.1 μM and 1.8 μM, respectively[6]. Tamoxifen activates autophagy and induces apoptosis[5]. Tamoxifen also can induce gene knockout of CreER(T2) transgenic mouse[7]. |
| Name | Tamoxifen-d3 |
|---|
| Description | Tamoxifen-d3 is the deuterium labeled Tamoxifen[1]. Tamoxifen (ICI 47699) is an orally active, selective estrogen receptor modulator (SERM) which blocks estrogen action in breast cells and can activate estrogen activity in other cells, such as bone, liver, and uterine cells[2][3][4]. Tamoxifen is a potent Hsp90 activator and enhances the Hsp90 molecular chaperone ATPase activity. Tamoxifen also potent inhibits infectious EBOV Zaire and Marburg (MARV) with IC50 of 0.1 μM and 1.8 μM, respectively[6]. Tamoxifen activates autophagy and induces apoptosis[5]. Tamoxifen also can induce gene knockout of CreER(T2) transgenic mouse[7]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
[2]. Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998 Nov 26;339(22):1609-18. [8]. Feil S, et, al. Inducible Cre mice. Methods Mol Biol. 2009530:343-63. |
| Molecular Formula | C26H26D3NO |
|---|---|
| Molecular Weight | 374.53 |