Canrenone
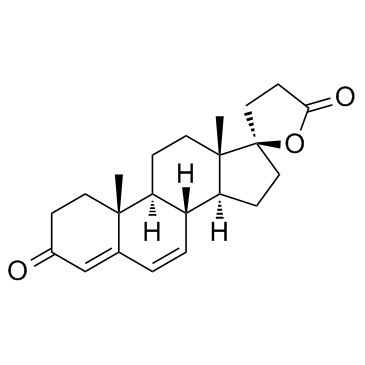
Canrenone structure
|
Common Name | Canrenone | ||
|---|---|---|---|---|
| CAS Number | 976-71-6 | Molecular Weight | 340.456 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 541.1±50.0 °C at 760 mmHg | |
| Molecular Formula | C22H28O3 | Melting Point | 158-160ºC | |
| MSDS | Chinese USA | Flash Point | 237.6±30.2 °C | |
| Symbol |


GHS08, GHS09 |
Signal Word | Warning | |
Use of CanrenoneCanrenone (Aldadiene; SC9376; SC14266) is an aldosterone antagonist extensively used as a diuretic agent. |
| Name | Canrenone |
|---|---|
| Synonym | More Synonyms |
| Description | Canrenone (Aldadiene; SC9376; SC14266) is an aldosterone antagonist extensively used as a diuretic agent. |
|---|---|
| Related Catalog | |
| Target |
Target: Aldosterone[1] |
| In Vitro | Canrenone inhibits the production of eortieosterone, 18-hydroxydesoxyeortieosterone, 18-hydroxycorticosterone and aldosterone in a dose-dependent manner[1]. Canrenone dose-dependently reduces platelet-derived growth factor–induced cell proliferation and motility. Canrenone inhibits the activity of the Na+/H+ exchanger 1 induced by platelet-derived growth factor[2]. |
| In Vivo | Canrenone is the principal active metabolite of Spironolactone in the rat only for a limited period. During chronic treatment a difference developed between the effect of Spironolactone and Canrenone on the RAAS indicating a decrease in the anti-mineralocorticoid activity of Canrenone and an increase in the efficacy of Spironolactone[3]. |
| Cell Assay | Confluent Hepatic Stellate Cells (HSC) are incubated in SFIF medium for 24 hours and exposed to increasing concentrations of canrenone (1, 5, 10, 25 μM). Cell viability is evaluated by the trypan blue dye exclusion test at the end of a 24- to 48-hour incubation period[2]. |
| Animal Admin | Rats[3] Canrenone (CAN) is given orally in two different doses (10.25, 20.5 mg/mL) to Male SPF Sprague-Dawley rats for 6 weeks. To determine the Na+, K+, fluid and aldosterone excretion the urine of the rats destined to be killed after 6 weeks is collected at weekly intervals[3]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 541.1±50.0 °C at 760 mmHg |
| Melting Point | 158-160ºC |
| Molecular Formula | C22H28O3 |
| Molecular Weight | 340.456 |
| Flash Point | 237.6±30.2 °C |
| Exact Mass | 340.203857 |
| PSA | 43.37000 |
| LogP | 2.99 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.581 |
| InChIKey | UJVLDDZCTMKXJK-WNHSNXHDSA-N |
| SMILES | CC12CCC(=O)C=C1C=CC1C2CCC2(C)C1CCC21CCC(=O)O1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 7 | |
|---|---|
| DownStream 6 | |
|
Aldosterone receptor blockers spironolactone and canrenone: two multivalent drugs.
Expert Opin. Pharmacother. 15(7) , 909-12, (2014) Canrenone is a derivative of spironolactone with lower antiandrogen activity. The drug is used only in few countries and can block all the side effects of aldosterone (ALDO). The drug is effective eve... |
|
|
Additional use of an aldosterone antagonist in patients with mild to moderate chronic heart failure: a systematic review and meta-analysis.
Br. J. Clin. Pharmacol. 75(5) , 1202-12, (2013) Aldosterone antagonists (AldoAs) have been used to treat severe chronic heart failure (CHF). There is uncertainty regarding the efficacy of using AldoAs in mild to moderate CHF with New York Heart Ass... |
|
|
The effects of canrenone on inflammatory markers in patients with metabolic syndrome.
Ann. Med. 47(1) , 47-52, (2015) To evaluate the effects of canrenone compared to placebo on blood pressure control, some non-conventional biomarkers in cardiovascular stratification, and on metalloproteinases in patients affected by... |
| Phanurane |
| Luvion |
| ALDADIENE |
| (17a)-17-Hydroxy-3-oxopregna-4,6-diene-21-carboxylic acid g-lactone |
| 17a-(2-Carboxyethyl)-17b-hydroxyandrosta-4,6-dien-3-one Lactone |
| Canrenone |
| Spiro[17H-cyclopenta[a]phenanthrene-17,2'(5'H)-furan]-3,5'(2H)-dione, 1,3',4',8,9,10,11,12,13,14,15,16-dodecahydro-10,13-dimethyl-, (8R,9S,10R,13S,14S,17R)- |
| 17a-(2-Carboxyethyl)-17b-hydroxy-3-oxoandrosta-4,6-diene Lactone |
| 3-(3-Oxo-17b-hydroxy-4,6-androstadien-17a-yl)propionic Acid g-Lactone |
| Contaren |
| 6-Dehydrotestosterone-17a-propionic Acid g-Lactone |
| CANRENONE-D4 |
| (8R,9S,10R,13S,14S,17R)-10,13-Dimethyl-1,8,9,10,11,12,13,14,15,16-decahydro-3'H-spiro[cyclopenta[a]phenanthrene-17,2'-furan]-3,5'(2H,4'H)-dione |
| 11614 R.P. |
 CAS#:38753-76-3
CAS#:38753-76-3 CAS#:976-70-5
CAS#:976-70-5 CAS#:52-01-7
CAS#:52-01-7 CAS#:434-03-7
CAS#:434-03-7![5'-hydroxyspiro[pregnane-17,2'-tetrahydrofuran]-5-en-3-one Structure](https://image.chemsrc.com/caspic/151/121936-43-4.png) CAS#:121936-43-4
CAS#:121936-43-4 CAS#:74-88-4
CAS#:74-88-4 CAS#:13934-61-7
CAS#:13934-61-7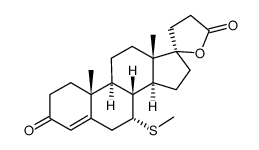 CAS#:38753-77-4
CAS#:38753-77-4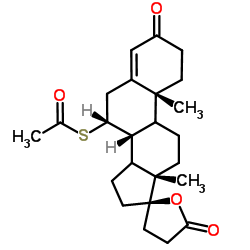 CAS#:33784-05-3
CAS#:33784-05-3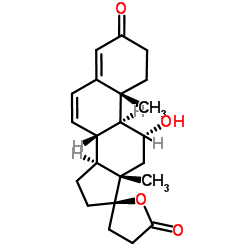 CAS#:192569-17-8
CAS#:192569-17-8![(1R,8S,9S,10R,11R,13S,14S,17R)-1,11-dihydroxy-10,13-dimethyl-1,3',4',8,9,10,11,12,13,14,15,16-dodecahydro-5'H-spiro[cyclopenta[a]phenanthrene-17,2'-furan]-3,5'(2H)-dione structure](https://image.chemsrc.com/caspic/458/1363502-03-7.png) CAS#:1363502-03-7
CAS#:1363502-03-7 CAS#:76676-33-0
CAS#:76676-33-0
