2-Acetylcyclohexanone

2-Acetylcyclohexanone structure
|
Common Name | 2-Acetylcyclohexanone | ||
|---|---|---|---|---|
| CAS Number | 874-23-7 | Molecular Weight | 140.180 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 224.3±0.0 °C at 760 mmHg | |
| Molecular Formula | C8H12O2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 79.4±0.0 °C | |
| Name | 2-Acetylcyclohexanone |
|---|---|
| Synonym | More Synonyms |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 224.3±0.0 °C at 760 mmHg |
| Molecular Formula | C8H12O2 |
| Molecular Weight | 140.180 |
| Flash Point | 79.4±0.0 °C |
| Exact Mass | 140.083725 |
| PSA | 34.14000 |
| LogP | 0.08 |
| Vapour Pressure | 0.1±0.4 mmHg at 25°C |
| Index of Refraction | 1.464 |
| Personal Protective Equipment | Eyeshields;Gloves;half-mask respirator (US);multi-purpose combination respirator cartridge (US) |
|---|---|
| Safety Phrases | S23-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2914299000 |
| Precursor 8 | |
|---|---|
| DownStream 8 | |
| HS Code | 2914299000 |
|---|---|
| Summary | 2914299000. other cyclanic, cyclenic or cyclotherpenic ketones without other oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
Tautomerization of 2-acetylcyclohexanone. 1. Characterization of keto-enol/enolate equilibria and reaction rates in water.
J. Org. Chem. 68(7) , 2680-8, (2003) The keto-enol tautomerism of 2-acetylcyclohexanone (ACHE) was studied in water under different experimental conditions. By contrast with other previously studied beta-diketones, the keto-enol intercon... |
|
|
Copper-catalyzed amination of (bromophenyl)ethanolamine for a concise synthesis of aniline-containing analogues of NMDA NR2B antagonist ifenprodil.
Org. Biomol. Chem. 8(5) , 1111-20, (2010) An operationally simple and concise synthesis of anilinoethanolamines, as NMDA NR2B receptor antagonist ifenprodil analogues, was developed via a copper-catalyzed amination of the corresponding bromoa... |
|
|
An alternative to the classical α-arylation: the transfer of an intact 2-iodoaryl from ArI(O₂CCF₃)₂.
Angew. Chem. Int. Ed. Engl. 53(42) , 11298-301, (2014) The α-arylation of carbonyl compounds is generally accomplished under basic conditions, both under metal catalysis and via aryl transfer from the diaryl λ(3)-iodanes. Here, we describe an alternative ... |
| 2-Acetylcyclohexanone |
| 2-acetylcyclohexanone for synthesis |
| 2-Acetyl-1-cyclohexanone |
| 2-acetylcyclohexa |
| 2-acetyl cyclohexanone |
| Cyclohexanone, 2-acetyl- |
| MFCD00001633 |
| EINECS 212-858-5 |
| cyclohexanone,2-acetyl- |
| 2-acetylcyclohexanon |
 CAS#:108-94-1
CAS#:108-94-1 CAS#:108-24-7
CAS#:108-24-7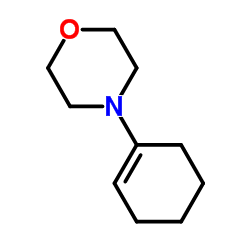 CAS#:670-80-4
CAS#:670-80-4 CAS#:75-36-5
CAS#:75-36-5 CAS#:1424-22-2
CAS#:1424-22-2 CAS#:6651-36-1
CAS#:6651-36-1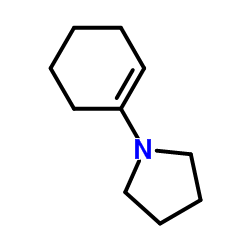 CAS#:1125-99-1
CAS#:1125-99-1 CAS#:17216-03-4
CAS#:17216-03-4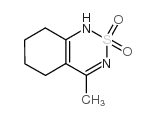 CAS#:3580-37-8
CAS#:3580-37-8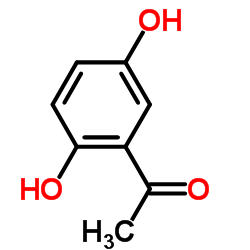 CAS#:490-78-8
CAS#:490-78-8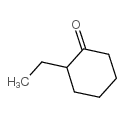 CAS#:4423-94-3
CAS#:4423-94-3 CAS#:463-51-4
CAS#:463-51-4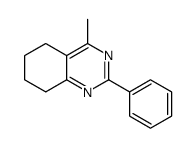 CAS#:142965-56-8
CAS#:142965-56-8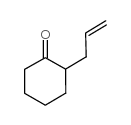 CAS#:94-66-6
CAS#:94-66-6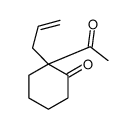 CAS#:67679-11-2
CAS#:67679-11-2
