JB-11 isethionate
Modify Date: 2025-08-31 12:24:15
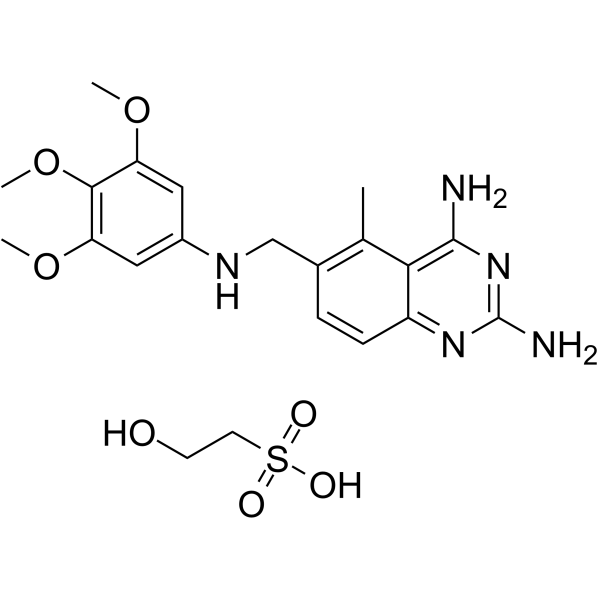
JB-11 isethionate structure
|
Common Name | JB-11 isethionate | ||
|---|---|---|---|---|
| CAS Number | 82935-04-4 | Molecular Weight | 495.54900 | |
| Density | N/A | Boiling Point | 647ºC at 760mmHg | |
| Molecular Formula | C21H29N5O7S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 345.1ºC | |
Use of JB-11 isethionateTrimetrexate (CI-898) isethionate is an antibiotic, also a potent and orally active dihydrofolate reductase (DHFR) inhibitor, reducing the production of DNA and RNA precursors and leading to cell death, with IC50 values of 4.74 nM and 1.35 nM for human DHFR and Toxoplasma gondii DHFR. Trimetrexate isethionate can also inhibit the growth of various cancer cells. Trimetrexate isethionate can be used for researching Pneumocystis carinii pneumonia (PCP) and cancer[1][2][3][4][5]. |
| Name | Ethanesulfonic acid, 2-hydroxy-, compd. with 5-methyl-6-[[(3,4,5-trimethoxyphenyl)amino]methyl]-2,4-quinazolinediamine (1_1) |
|---|
| Description | Trimetrexate (CI-898) isethionate is an antibiotic, also a potent and orally active dihydrofolate reductase (DHFR) inhibitor, reducing the production of DNA and RNA precursors and leading to cell death, with IC50 values of 4.74 nM and 1.35 nM for human DHFR and Toxoplasma gondii DHFR. Trimetrexate isethionate can also inhibit the growth of various cancer cells. Trimetrexate isethionate can be used for researching Pneumocystis carinii pneumonia (PCP) and cancer[1][2][3][4][5]. |
|---|---|
| Related Catalog | |
| Target |
Toxoplasma gondii |
| In Vitro | Trimetrexate isethionate (0.1 μM; 18 h) completely inhibits proliferation of toxoplasma in murine macrophages[3]. Trimetrexate isethionate (1 μM) can cross the toxoplasma cell membrane and rapidly reaches high intracellular concentrations (108 pmol/107 cells within 10 min) [3]. Trimetrexate (0.1 mM; 24 h) inhibits cell growth by 50-60% in SNU-C4 and NCI-H630 cell lines[5]. Trimetrexate (1 and 10 mM; 24 h) produces lethality and inhibits DHFR in C4 cells[5]. Cell Proliferation Assay[5] Cell Line: SNU-C4 and NCI-H630 Concentration: 0.1 mM Incubation Time: 24 h Result: Inhibited cell growth by 50-60% in both cell lines. Cell Proliferation Assay[5] Cell Line: C4 cells Concentration: 1 and 10 mM Incubation Time: 24 h Result: Produced 42% and 50% lethality at 1 and 10 mM, respectively. |
| In Vivo | Trimetrexate (180 mg/kg or 30 mg/kg; p.o. or i.p.; daily) isethionate extends the median survival of the toxoplasma infected mice and shows antitoxoplasma activity[3]. Trimetrexate (0-30 mg/kg; i.v.; once daily for 5days) isethionate shows chronic toxicity in rats[4]. Animal Model: Toxoplasma infected female BALB/c mice weighing about 20 g[3] Dosage: 180 mg/kg or 30 mg/kg Administration: 180 mg/kg per day orally in the drinking water or 30 mg/kg per day i.p. Result: Extended the median survival of the infected mice to 10 d (p.o.) or 19 d (i.p.). Animal Model: Charles River Wistar Crl(WI)BR rats weighing approximately 150 to 200 g[4] Dosage: 0, 1, 10, or 30 mg/kg Administration: Intravenous injection, once daily for 5 consecutive days followed by a 23-day recovery period Result: Showed chronic toxicity, the testicular changes persisting during the course of multiple cycles of dosing were not reversible within 21 days, but required an additional 56 days for essentially complete recovery. |
| References |
[2]. Fulton, B., et al. Trimetrexate. Drugs 49, 563–576 (1995). |
| Boiling Point | 647ºC at 760mmHg |
|---|---|
| Molecular Formula | C21H29N5O7S |
| Molecular Weight | 495.54900 |
| Flash Point | 345.1ºC |
| Exact Mass | 495.17900 |
| PSA | 200.52000 |
| LogP | 3.92320 |
| InChIKey | YATKEMOVGUXIDY-UHFFFAOYSA-N |
| SMILES | COc1cc(NCc2ccc3nc(N)nc(N)c3c2C)cc(OC)c1OC.O=S(=O)(O)CCO |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|