cefpodoxime
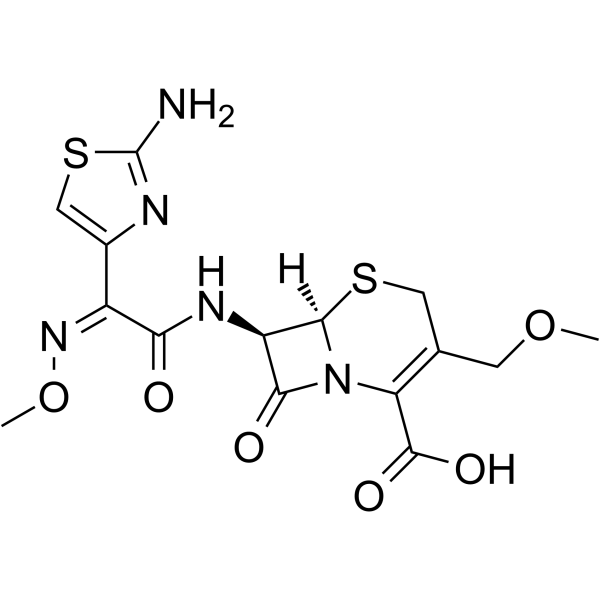
cefpodoxime structure
|
Common Name | cefpodoxime | ||
|---|---|---|---|---|
| CAS Number | 80210-62-4 | Molecular Weight | 427.455 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C15H17N5O6S2 | Melting Point | 200-202ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of cefpodoximeCefpodoxime (Cefpodoxime acid) is a potent antibiotic active against gram-positive and gram-negative bacteria. Cefpodoxime inhibits the majority of cells in microbial populations. Cefpodoxime can be used for acute otitis media, sinusitis and tosillopharyngitis research[1][2]. |
| Name | cefpodoxime |
|---|---|
| Synonym | More Synonyms |
| Description | Cefpodoxime (Cefpodoxime acid) is a potent antibiotic active against gram-positive and gram-negative bacteria. Cefpodoxime inhibits the majority of cells in microbial populations. Cefpodoxime can be used for acute otitis media, sinusitis and tosillopharyngitis research[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Cefpodoxime (Cefpodoxime acid) inhibits gram-negative anaerobic rods (Bacteroidaceae) with MIC values of 0.125-4 mg/L. Cefpodoxime inhibits Veillonella parvula with MIC values of 0.25-8 mg/L. Cefpodoxime inhibits Peptostreptococcus micros, Peptostreptococcus asaccharolyticus and Ruminococcus bromii with MIC values of <2 mg/L[1]. Cefpodoxime (Cefpodoxime acid) inhibits bacterial populations of S. pneumoniae and S. pyogenes. cfu[2]. |
| In Vivo | Cephalosporins (2.5-50 mg/kg; p.o.; every 8 hours; for 48 hours) have good curative effect in mice[3]. Animal Model: Female Swiss CD1 mice[3] Dosage: 2.5, 5, 10, 25, 40 and 50 mg/kg Administration: Oral administration; every 8 hours; for 48 hours Result: Efficacy was obtained with values of >350. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Melting Point | 200-202ºC |
| Molecular Formula | C15H17N5O6S2 |
| Molecular Weight | 427.455 |
| Exact Mass | 427.062012 |
| PSA | 209.98000 |
| LogP | 0.94 |
| Index of Refraction | 1.780 |
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H317-H334 |
| Precautionary Statements | P261-P280-P342 + P311 |
| Hazard Codes | Xn |
| Risk Phrases | 42/43 |
| Safety Phrases | 22-36/37-45 |
| RIDADR | NONH for all modes of transport |
|
~% 
cefpodoxime CAS#:80210-62-4 |
| Literature: WO2011/77217 A1, ; Page/Page column 9 ; |
|
~% 
cefpodoxime CAS#:80210-62-4 |
| Literature: WO2013/41999 A1, ; |
|
~% 
cefpodoxime CAS#:80210-62-4 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 37, # 9 p. 2369 - 2374 |
|
Isolation of Escherichia coli strains with AcrAB-TolC efflux pump-associated intermediate interpretation or resistance to fluoroquinolone, chloramphenicol and aminopenicillin from dogs admitted to a university veterinary hospital.
J. Vet. Med. Sci. 76(7) , 937-45, (2014) Understanding the prevalence of antimicrobial-resistance and the relationship between emergence of resistant bacteria and clinical treatment can facilitate design of effective treatment strategies. We... |
|
|
Increase in resistance to extended-spectrum cephalosporins in Salmonella isolated from retail chicken products in Japan.
PLoS ONE 10(2) , e0116927, (2015) Extended-spectrum β-lactamase (ESBL)-producing Salmonella are one of the most important public health problems in developed countries. ESBL-producing Salmonella strains have been isolated from humans ... |
|
|
Cephalosporinases associated with outer membrane vesicles released by Bacteroides spp. protect gut pathogens and commensals against β-lactam antibiotics.
J. Antimicrob. Chemother. 70(3) , 701-9, (2015) To identify β-lactamase genes in gut commensal Bacteroides species and to assess the impact of these enzymes, when carried by outer membrane vesicles (OMVs), in protecting enteric pathogens and commen... |
| (6R,7R)-7-({(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(methyloxy)imino]acetyl}amino)-3-[(methyloxy)methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| Cefpodoxime Acid |
| 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2Z)-2-(2-amino-4-thiazolyl)-2-(methoxyimino)-1-oxoethyl]amino]-3-(methoxymethyl)-8-oxo-, (6R,7R)- |
| (6R,7R)-7-{[(2Z)-2-(2-Amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-3-(methoxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| cefpodoxime |
| cefpodoxima |
| U 76253A |
| (6R-(6a,7b(Z)))-7-(((2-amino-4-thiazolyl)(methoxyimino)acetyl)amino)-3-(methoxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid |
| Cefpodoxime Proxetil EP Impurity A |
| Cefpodoxime Proxetil Impurity 1 |
![(pivaloyloxy)methyl (6R,7R)-7-((Z)-2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-3-(methoxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate structure](https://www.chemsrc.com/caspic/114/82618-98-2.png)
 CAS#:87239-81-4
CAS#:87239-81-4
