Daltroban
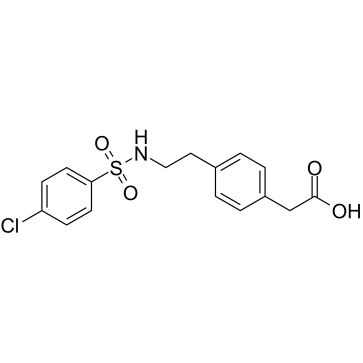
Daltroban structure
|
Common Name | Daltroban | ||
|---|---|---|---|---|
| CAS Number | 79094-20-5 | Molecular Weight | 353.82100 | |
| Density | 1.378g/cm3 | Boiling Point | 555.3ºC at 760 mmHg | |
| Molecular Formula | C16H16ClNO4S | Melting Point | 132.5-137.4 °C(lit.) | |
| MSDS | USA | Flash Point | 289.6ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of DaltrobanDaltroban (BM-13505) is a selective and specific thromboxane A2 (TXA2) receptor antagonist. Daltroban increase intracellular calcium in vascular smooth muscle cells. Daltroban shows protective effect in reperfusion injury[1][2]. |
| Name | Daltroban |
|---|---|
| Synonym | More Synonyms |
| Description | Daltroban (BM-13505) is a selective and specific thromboxane A2 (TXA2) receptor antagonist. Daltroban increase intracellular calcium in vascular smooth muscle cells. Daltroban shows protective effect in reperfusion injury[1][2]. |
|---|---|
| Related Catalog | |
| Target |
TXA2 |
| In Vivo | Daltroban (BM-13505) (1 mg/kg; i.v.; per hour) exerts protective effect in reperfusion injury following acute myocardial ischemia in cats[2]. In comparison with vehicle (physiological saline)-treated cats, Daltroban (20 mg/kg per hour i.v.) reduces the ischaemia-induced rise in the ST segment and prevented the development of a Q-wave in the ECG during reperfusion. Daltroban protects the myocardium from ischaemic injury and that this effect involves prevention of ischaemia-induced leukocytosis[3]. Animal Model: Adult male cats (2.8 to 4.6 kg; anesthetized cat model)[2] Dosage: 1 mg/kg Administration: i.v.; 30 minutes before reperfusion at a rate of 1 mg/kg followed by 1 mg/kg/hour Result: Significantly reduced the area of ischemic tissue as a percent of total left ventricular mass and total area at risk, without altering basic hemodynamics and thereby not influencing myocardial oxygen demand. |
| References |
| Density | 1.378g/cm3 |
|---|---|
| Boiling Point | 555.3ºC at 760 mmHg |
| Melting Point | 132.5-137.4 °C(lit.) |
| Molecular Formula | C16H16ClNO4S |
| Molecular Weight | 353.82100 |
| Flash Point | 289.6ºC |
| Exact Mass | 353.04900 |
| PSA | 91.85000 |
| LogP | 3.95980 |
| Appearance of Characters | white |
| Vapour Pressure | 3.63E-13mmHg at 25°C |
| Index of Refraction | 1.609 |
| InChIKey | IULOBWFWYDMECP-UHFFFAOYSA-N |
| SMILES | O=C(O)Cc1ccc(CCNS(=O)(=O)c2ccc(Cl)cc2)cc1 |
| Water Solubility | DMSO: 22 mg/mL, soluble |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
~% 
Daltroban CAS#:79094-20-5 |
| Literature: European Journal of Medicinal Chemistry, , vol. 26, # 8 p. 821 - 827 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
|
Cyclooxygenase-2 inhibition and thromboxane A(2) receptor antagonism attenuate hypoxic pulmonary vasoconstriction in a porcine model.
Acta Physiol. (Oxf.) 205(4) , 507-19, (2012) Hypoxic pulmonary vasoconstriction (HPV) causes pulmonary hypertension that may lead to right heart failure. We hypothesized that the COX-2 inhibitor nimesulide and the thromboxane A(2) receptor antag... |
|
|
Prostaglandin endoperoxides and thromboxane A2 activate the same receptor isoforms in human platelets.
Thromb. Haemost. 87(1) , 114-21, (2002) Arachidonic acid (AA) is a potent inducer of platelet aggregation in vitro; this activity is due to its conversion to biologically active metabolites, prostaglandin (PG) endoperoxides and thromboxane ... |
|
|
Increase by anaphylatoxin C5a of glucose output in perfused rat liver via prostanoids derived from nonparenchymal cells: direct action of prostaglandins and indirect action of thromboxane A(2) on hepatocytes.
Hepatology 30 , 454-461, (1999) In the perfused rat liver the anaphylatoxin C5a enhanced glucose output, reduced flow, and elevated prostanoid overflow. Because hepatocytes (HCs) do not express C5a receptors, the metabolic C5a actio... |
| 4-[2-(4-Chlorobenzene-sulfonamido)ethyl]benzeneacetic acid |
| [4-[2-(4-chlorobenzenesulfonamido)ethyl]phenyl]acetic acid |
| 2-[4-[2-(4-chlorophenylsulphonylamino)-ethyl]-phenyl]-acetic acid |
| 4-[2-[(4-CHLOROPHENYLSULFONYL)AMINO]ETHYL]BENZENEACETIC ACID |
| DatelliptiumChloride |
| 2-[4-[2-(4-chlorophenyl)sulfonylaminoethyl]phenyl]acetic acid |
| 4-[2-(4-chlorobenzenesulphonamido)-ethyl]-phenylacetic acid |
| 4-((((4-chlorophenyl)sulfonyl)amino)ethyl)benzene acetic acid |
| MFCD00868295 |