Triparanol
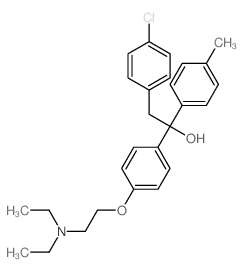
Triparanol structure
|
Common Name | Triparanol | ||
|---|---|---|---|---|
| CAS Number | 78-41-1 | Molecular Weight | 438.00100 | |
| Density | 1.133g/cm3 | Boiling Point | 546.9ºC at 760 mmHg | |
| Molecular Formula | C27H32ClNO2 | Melting Point | 102.9-103.7ºC | |
| MSDS | Chinese USA | Flash Point | 284.5ºC | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
Use of TriparanolTriparanol (MER-29) is a potent cholesterol biosynthesis inhibitor blocking the 24-dehydrocholesterol reductase (24-DHCR). Triparanol can interfere with posttranslational modification of Hedgehog signaling molecules as well as the sterol sensing domain of its receptor PTCH1, leading to down-regulation of Hh signaling. Triparanol suppresses human tumor growth[1][2]. |
| Name | 2-(4-chlorophenyl)-1-[4-[2-(diethylamino)ethoxy]phenyl]-1-(4-methylphenyl)ethanol |
|---|---|
| Synonym | More Synonyms |
| Description | Triparanol (MER-29) is a potent cholesterol biosynthesis inhibitor blocking the 24-dehydrocholesterol reductase (24-DHCR). Triparanol can interfere with posttranslational modification of Hedgehog signaling molecules as well as the sterol sensing domain of its receptor PTCH1, leading to down-regulation of Hh signaling. Triparanol suppresses human tumor growth[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Triparanol can block proliferation and induce apoptosis in multiple human cancer cells including lung, breast, liver, pancreatic, prostate cancer and melanoma cells, and growth inhibition can be rescued by exogenous addition of cholesterol[2]. |
| References |
[1]. AVIGAN J, et al. The mechanism of action of MER-29. Prog Cardiovasc Dis. 1960;2:525-530. |
| Density | 1.133g/cm3 |
|---|---|
| Boiling Point | 546.9ºC at 760 mmHg |
| Melting Point | 102.9-103.7ºC |
| Molecular Formula | C27H32ClNO2 |
| Molecular Weight | 438.00100 |
| Flash Point | 284.5ºC |
| Exact Mass | 437.21200 |
| PSA | 32.70000 |
| LogP | 5.84760 |
| Index of Refraction | 1.582 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H318 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | 22-41 |
| RIDADR | NONH for all modes of transport |
| RTECS | KK2400000 |
|
Transcriptional Regulation of Cytosolic Sulfotransferase 1C2 by Intermediates of the Cholesterol Biosynthetic Pathway in Primary Cultured Rat Hepatocytes.
J. Pharmacol. Exp. Ther. 355 , 429-41, (2015) Cytosolic sulfotransferase 1C2 (SULT1C2) is expressed in the kidney, stomach, and liver of rats; however, the mechanisms regulating expression of this enzyme are not known. We evaluated transcriptiona... |
|
|
Teratogen-mediated inhibition of target tissue response to Shh signaling.
Science 280(5369) , 1603-7, (1998) Veratrum alkaloids and distal inhibitors of cholesterol biosynthesis have been studied for more than 30 years as potent teratogens capable of inducing cyclopia and other birth defects. Here, it is sho... |
|
|
Triggering of Erythrocyte Death by Triparanol.
Toxins (Basel.) 7 , 3359-71, (2015) The cholesterol synthesis inhibitor Triparanol has been shown to trigger apoptosis in several malignancies. Similar to the apoptosis of nucleated cells, erythrocytes may enter eryptosis, the suicidal ... |
| Metasqualene |
| MER 29 |
| EINECS 201-115-0 |
| triparanolum |
| valip |
| TRIPARANOL |