Ethyl 5-hydroxy-2-methyl-1H-indole-3-carboxylate
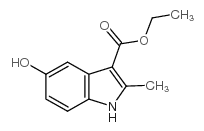
Ethyl 5-hydroxy-2-methyl-1H-indole-3-carboxylate structure
|
Common Name | Ethyl 5-hydroxy-2-methyl-1H-indole-3-carboxylate | ||
|---|---|---|---|---|
| CAS Number | 7598-91-6 | Molecular Weight | 219.23700 | |
| Density | 1.282 g/cm3 | Boiling Point | 406.7ºC at 760 mmHg | |
| Molecular Formula | C12H13NO3 | Melting Point | 205-208ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 199.7ºC | |
Use of Ethyl 5-hydroxy-2-methyl-1H-indole-3-carboxylateEthyl 5-hydroxy-2-methyl-1H-indole-3-carboxylate is an active compound, and can be used in the synthesis of 2-phenylthiomethyl-indole derivatives, 2-phenylthiomethyl-indole derivative is a 5-lipoxygenase (5-LO) inhibitor[1]. |
| Name | ethyl 5-hydroxy-2-methylindole-3-carboxylate |
|---|---|
| Synonym | More Synonyms |
| Description | Ethyl 5-hydroxy-2-methyl-1H-indole-3-carboxylate is an active compound, and can be used in the synthesis of 2-phenylthiomethyl-indole derivatives, 2-phenylthiomethyl-indole derivative is a 5-lipoxygenase (5-LO) inhibitor[1]. |
|---|---|
| Related Catalog |
| Density | 1.282 g/cm3 |
|---|---|
| Boiling Point | 406.7ºC at 760 mmHg |
| Melting Point | 205-208ºC(lit.) |
| Molecular Formula | C12H13NO3 |
| Molecular Weight | 219.23700 |
| Flash Point | 199.7ºC |
| Exact Mass | 219.09000 |
| PSA | 62.32000 |
| LogP | 2.35860 |
| Index of Refraction | 1.5270 (estimate) |
|
~78% 
Ethyl 5-hydroxy... CAS#:7598-91-6 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 43, # 4 p. 873 - 879 |
|
~88% 
Ethyl 5-hydroxy... CAS#:7598-91-6 |
| Literature: Research on Chemical Intermediates, , vol. 36, # 8 p. 975 - 983 |
| Precursor 3 | |
|---|---|
| DownStream 7 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Discovery of 4-amino and 4-hydroxy-1-aroylindoles as potent tubulin polymerization inhibitors.
J. Med. Chem. 51 , 4351, (2008) 1-Aroylindoline, 1-aroyl-1,2,3,4-tetrahydroquinoline, and 1-aroylindole derivatives were synthesized and evaluated for anticancer activity. The 4-amino and 4-hydroxy-1-aroylindoles 26 and 27 with IC 5... |
|
|
Synthesis and pharmacology of benzoxazines as highly selective antagonists at M(4) muscarinic receptors.
J. Med. Chem. 45 , 3094, (2002) Previously, we reported on PD 102807 (41) as being the most selective synthetic M(4) muscarinic antagonist identified to date. Synthesized analogues of 41 showed no improvement in affinity and selecti... |
|
|
5-hydroxyindole-2-carboxylic acid amides: novel histamine-3 receptor inverse agonists for the treatment of obesity.
J. Med. Chem. 52 , 3855, (2009) Obesity is a major risk factor in the development of conditions such as hypertension, hyperglycemia, dyslipidemia, coronary artery disease, and cancer. Several pieces of evidence across different spec... |
| MFCD00044575 |
| ethyl 5-hydroxy-2-methyl-1H-indole-3-carboxylate |
| EINECS 231-507-7 |
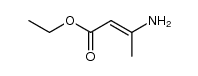
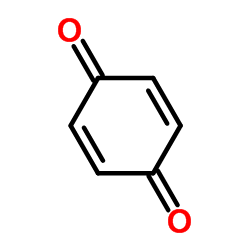
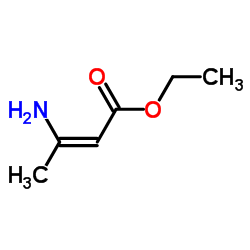
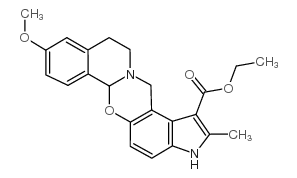 CAS#:23062-91-1
CAS#:23062-91-1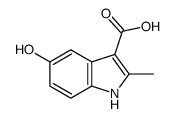 CAS#:71982-15-5
CAS#:71982-15-5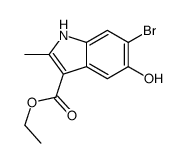 CAS#:16052-67-8
CAS#:16052-67-8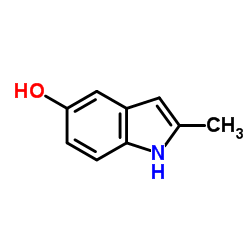 CAS#:13314-85-7
CAS#:13314-85-7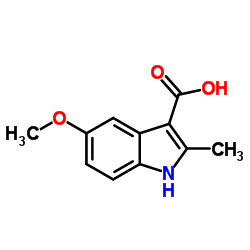 CAS#:32387-22-7
CAS#:32387-22-7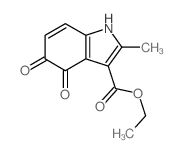 CAS#:50995-59-0
CAS#:50995-59-0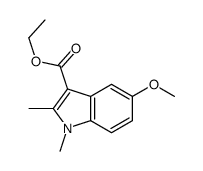 CAS#:40963-98-2
CAS#:40963-98-2
