| Structure | Name/CAS No. | Articles |
|---|---|---|
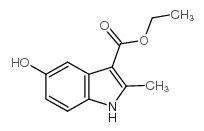 |
Ethyl 5-hydroxy-2-methyl-1H-indole-3-carboxylate
CAS:7598-91-6 |
|
 |
Colchicine
CAS:64-86-8 |