Lovastatin

Lovastatin structure
|
Common Name | Lovastatin | ||
|---|---|---|---|---|
| CAS Number | 75330-75-5 | Molecular Weight | 404.540 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 559.2±50.0 °C at 760 mmHg | |
| Molecular Formula | C24H36O5 | Melting Point | 175°C | |
| MSDS | Chinese USA | Flash Point | 185.3±23.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of LovastatinLovastatin is a cell-permeable HMG-CoA reductase inhibitor used to lower cholesterol. |
| Name | lovastatin |
|---|---|
| Synonym | More Synonyms |
| Description | Lovastatin is a cell-permeable HMG-CoA reductase inhibitor used to lower cholesterol. |
|---|---|
| Related Catalog | |
| Target |
HMG-CoA reductase[1] |
| In Vitro | Lovastatin is an inactive lactone prodrug that must be chemically or enzymatically converted to its dihydroxy open-acid form in order to elicit inhibitory activity. Lovastatin in its hydroxy acid form is an exceptionally potent competitive inhibitor of liver HMG CoA reductase[1]. Lovastatin, other than its anticholesterol property, has diverse applications in the field of osteoporosis, neuro-degeneration, rheumatoid arthritis, antifungals and also is reported to reduce proliferation of lung cancer cells, breast cancer (MCF-7), liver cancer (HepG2). Lovastatin treatments show significant dose dependent cytotoxic effect on HeLa cells with IC50 value of 160 μg/mL. Lovastatin is effective to accelerate hydroxyl radical scavenging activity (54.06%) at an IC50 of 3601 μg/mL[2]. |
| In Vivo | Lovastatin is an inactive lactone that is hydrolyzed in the liver to an active f3-hydroxyacid form. This principal metabolite is the inhibitor of the enzyme HMG-CoA reductase. The Ki is 1 nM. Lovastatin and its β-hydroxyacid metabolite are highly bound to human plasma proteins. Lovastatin crosses the blood-brain and placental barriers[3]. Lovastatin produces a profound reduction of apolipoprotein-B-containing lipoproteins, especially LDL cholesterol and, to a lesser extent, plasma triglycerides, and a small increase in HDL cholesterol[4]. |
| Cell Assay | Hela cells are treated with lovastatin (0, 5, 10, 20, 40, 80, 160, 320 μg/mL) for 24 h. Cells treated with culture medium serves as a negative control. cell viability is measured using the MTT based colorimetric assay [2]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 559.2±50.0 °C at 760 mmHg |
| Melting Point | 175°C |
| Molecular Formula | C24H36O5 |
| Molecular Weight | 404.540 |
| Flash Point | 185.3±23.6 °C |
| Exact Mass | 404.256287 |
| PSA | 72.83000 |
| LogP | 4.07 |
| Vapour Pressure | 0.0±3.4 mmHg at 25°C |
| Index of Refraction | 1.532 |
| InChIKey | PCZOHLXUXFIOCF-BXMDZJJMSA-N |
| SMILES | CCC(C)C(=O)OC1CC(C)C=C2C=CC(C)C(CCC3CC(O)CC(=O)O3)C21 |
| Storage condition | 2-8°C |
| Water Solubility | 0.0004 mg/mL at 25 ºC |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S24/25 |
| RIDADR | 3077 |
| WGK Germany | 3 |
| RTECS | EK7907000 |
| Packaging Group | III |
| Hazard Class | 9 |
| HS Code | 2932999099 |
| Precursor 2 | |
|---|---|
| DownStream 9 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Oxysterols synergize with statins by inhibiting SREBP-2 in ovarian cancer cells.
Gynecol. Oncol. 135(2) , 333-41, (2014) Determine mechanisms responsible for enhanced statin efficacy in a novel statin combination we name STOX (STatin-OXysterol).Ovarian cancer cell lines were treated with combinations of statins and oxys... |
|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
| MEVINOLIN |
| LOVALIP |
| (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2S)-2-methylbutanoate |
| (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl-(2S)-2-methylbutanoat |
| Lovastatin |
| 1,2,6,7,8,8a-Hexahydro-b,d-dihydroxy-2,6-dimethyl-8-(2-methyl-1-oxobutoxy)-1-naphthaleneheptanoic Acid d-Lactone |
| 2b,6a-Dimethyl-8a-(2-methyl-1-oxobutoxy)mevinic Acid Lactone |
| (+)-Mevinolin |
| Butanoic acid, 2-methyl-, (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester, (2S)- |
| (2S)-2-Methylbutanoic acid (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester |
| LOVASTIN |
| Altocor |
| MEVACOR |
| Antibiotic MB 530B |
| Altoprev |
| Rovacor |
| 6a-Methylcompactin |
| Sivlor |
| (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-Hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl (S)-2-Methylbutyrate |
| (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydro-1-naphthalenyl (2S)-2-methylbutanoate |
| MFCD00072164 |
| mevlor |
| [1S-[1a(R*),3a,7b,8b(2S*,4S*),8ab]]-2-Methylbutanoic Acid1,2,3,7,8,8a-Hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl]-1-naphthalenyl Ester |
| msd803 |
| Paschol |
| Simvastatin Impurity 5 |
![6(R)-[2-[1,2,6,7,8,8a(R)-Hexahydro-2(S),6(R)-dimethyl-8(S)-[[2(S)-methylbutyryl]oxy]-1(S)-naphtyl]ethyl]-3,4,5,6-tetrahydro-4(R)-[(tert-butyldimethylsilyl)oxy]-2H-pyran-2-one Structure](https://image.chemsrc.com/caspic/013/79691-11-5.png) CAS#:79691-11-5
CAS#:79691-11-5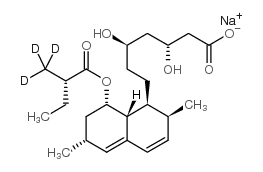 CAS#:75225-51-3
CAS#:75225-51-3![4-(TERT-BUTYLDIMETHYLSILANYLOXY)-6-[2-(8-HYDROXY-2,6-DIMETHYL-1,2,6,7,8,8A-HEXAHYDRONAPHTHALEN-1-YL)ETHYL]TETRAHYDROPYRAN-2-ONE structure](https://image.chemsrc.com/caspic/414/79902-31-1.png) CAS#:79902-31-1
CAS#:79902-31-1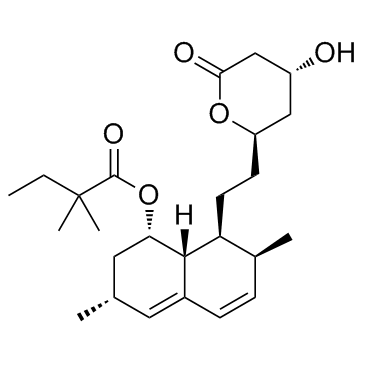 CAS#:79902-63-9
CAS#:79902-63-9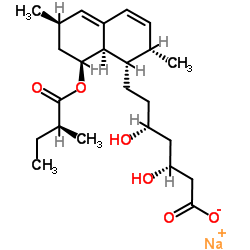 CAS#:75225-50-2
CAS#:75225-50-2 CAS#:67-64-1
CAS#:67-64-1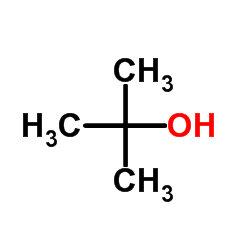 CAS#:75-65-0
CAS#:75-65-0 CAS#:109273-98-5
CAS#:109273-98-5 CAS#:151006-15-4
CAS#:151006-15-4 CAS#:132748-10-8
CAS#:132748-10-8
