CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
AN7600000
-
CHEMICAL NAME :
-
2',6'-Acetoxylidide, 2-(diethylamino)-, hydrochloride
-
CAS REGISTRY NUMBER :
-
73-78-9
-
LAST UPDATED :
-
199712
-
DATA ITEMS CITED :
-
27
-
MOLECULAR FORMULA :
-
C14-H22-N2-O.Cl-H
-
MOLECULAR WEIGHT :
-
270.84
-
WISWESSER LINE NOTATION :
-
2N2&1VMR B1 F1 &GH
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
Standard Draize test
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
REFERENCE :
-
AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. (Heymans Institute of Pharmacology, De Pintelaan 185, B-9000 Ghent, Belgium) V.4- 1898- Volume(issue)/page/year: 137,410,1962
-
TYPE OF TEST :
-
Standard Draize test
-
ROUTE OF EXPOSURE :
-
Administration into the eye
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
REFERENCE :
-
AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. (Heymans Institute of Pharmacology, De Pintelaan 185, B-9000 Ghent, Belgium) V.4- 1898- Volume(issue)/page/year: 137,410,1962 ** ACUTE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - infant
-
DOSE/DURATION :
-
1600 ug/kg/9H-I
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
AEMED3 Annals of Emergency Medicine. (American College of Emergency Physicians, 1125 Executive Circle, Irving, TX 75038) Volume(issue)/page/year: 17,725,1988
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - infant
-
DOSE/DURATION :
-
1632 mg/kg/1W-I
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
CPEDAM Clinical Pediatrics (Philadelphia). (Lippincott/Harper, Journal Fulfillment Dept., 2350 Virginia Ave., Hagerstown, MD 21740) V.1- 1962- Volume(issue)/page/year: 22,190,1983
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - infant
-
DOSE/DURATION :
-
10 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Behavioral - coma Lungs, Thorax, or Respiration - other changes
-
REFERENCE :
-
JTCTDW Journal of Toxicology, Clinical Toxicology. (Marcel Dekker, 270 Madison Ave., New York, NY 10016) V.19- 1982- Volume(issue)/page/year: 28,101,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
60 mg/kg/1H
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Vascular - BP lowering not characterized in autonomic section
-
REFERENCE :
-
JTCTDW Journal of Toxicology, Clinical Toxicology. (Marcel Dekker, 270 Madison Ave., New York, NY 10016) V.19- 1982- Volume(issue)/page/year: 24,51,1986
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
9 mg/kg/4H-C
-
TOXIC EFFECTS :
-
Cardiac - cardiomyopathy including infarction
-
REFERENCE :
-
DICPBB Drug Intelligence and Clinical Pharmacy. (POB 42435, Cincinnati, OH 45242) V.3- 1969- Volume(issue)/page/year: 19,669,1985
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
7143 ug/kg
-
TOXIC EFFECTS :
-
Cardiac - pulse rate increase, without fall in BP
-
REFERENCE :
-
CHETBF Chest. (American College of Chest Physicians, 911 Busse Hwy, Park Ridge, IL 60068) V.57- 1970- Volume(issue)/page/year: 61,682,1972
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Implant
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
5714 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
CMAJAX Canadian Medical Association Journal. (Canadian Medical Assoc., POB 8650, Ottawa, ON K1G 0G8, Canada) V.1- 1911- Volume(issue)/page/year: 137,219,1987
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
122 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JPETAB Journal of Pharmacology and Experimental Therapeutics. (Williams & Wilkins Co., 428 E. Preston St., Baltimore, MD 21202) V.1- 1909/10- Volume(issue)/page/year: 111,224,1954
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
570 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
RPOBAR Research Progress in Organic-Biological and Medicinal Chemistry. (New York, NY) V.1-3, 1964-72. Discontinued. Volume(issue)/page/year: 2,299,1970
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
21 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
RPOBAR Research Progress in Organic-Biological and Medicinal Chemistry. (New York, NY) V.1-3, 1964-72. Discontinued. Volume(issue)/page/year: 2,299,1970
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
220 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
RPOBAR Research Progress in Organic-Biological and Medicinal Chemistry. (New York, NY) V.1-3, 1964-72. Discontinued. Volume(issue)/page/year: 2,299,1970
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
63 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. (Heymans Institute of Pharmacology, De Pintelaan 185, B-9000 Ghent, Belgium) V.4- 1898- Volume(issue)/page/year: 274,253,1985
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
163 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
PSEBAA Proceedings of the Society for Experimental Biology and Medicine. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1903/04- Volume(issue)/page/year: 103,353,1960
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
15 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Lungs, Thorax, or Respiration - respiratory stimulation
-
REFERENCE :
-
JPPMAB Journal of Pharmacy and Pharmacology. (Pharmaceutical Soc. of Great Britain, 1 Lambeth High St., London SEI 7JN, UK) V.1- 1949- Volume(issue)/page/year: 14,48T,1962
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
177 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
DDREDK Drug Development Research. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1981- Volume(issue)/page/year: 21,277,1990
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
65700 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JNPHAG Journal de Pharmacologie. (SPPIF, B.P.22, F-41353 Vineuil, France) V.1- 1970- Volume(issue)/page/year: 2,240,1971
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
25600 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,879,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intratracheal
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
28 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. (Heymans Institute of Pharmacology, De Pintelaan 185, B-9000 Ghent, Belgium) V.4- 1898- Volume(issue)/page/year: 200,359,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
24500 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. (Heymans Institute of Pharmacology, De Pintelaan 185, B-9000 Ghent, Belgium) V.4- 1898- Volume(issue)/page/year: 113,313,1958
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Parenteral
-
SPECIES OBSERVED :
-
Amphibian - frog
-
DOSE/DURATION :
-
159 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. (Heymans Institute of Pharmacology, De Pintelaan 185, B-9000 Ghent, Belgium) V.4- 1898- Volume(issue)/page/year: 289,278,1987 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Implant
-
DOSE :
-
7500 mg/kg
-
SEX/DURATION :
-
female 3-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
ANESAV Anesthesiology. (Lippincott/Harper, Journal Fulfillment Dept., 2350 Virginia Ave., Hagerstown, MD 21740) V.1- 1940- Volume(issue)/page/year: 65,626,1986 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 83491 No. of Facilities: 397 (estimated) No. of Industries: 3 No. of Occupations: 5 No. of Employees: 13412 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 83491 No. of Facilities: 1385 (estimated) No. of Industries: 6 No. of Occupations: 17 No. of Employees: 68071 (estimated) No. of Female Employees: 56919 (estimated)
|

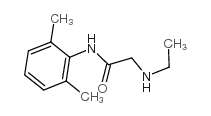 CAS#:7728-40-7
CAS#:7728-40-7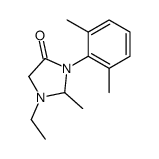 CAS#:32845-42-4
CAS#:32845-42-4