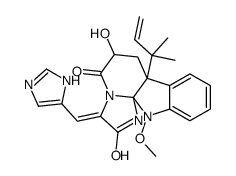
Meleagrin
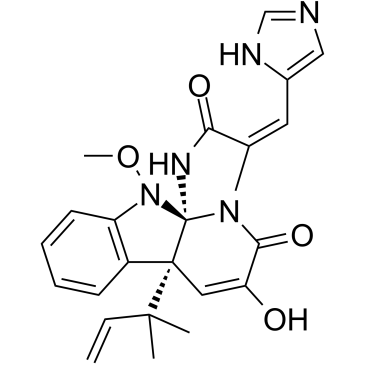
Meleagrin structure
|
Common Name | Meleagrin | ||
|---|---|---|---|---|
| CAS Number | 71751-77-4 | Molecular Weight | 433.46000 | |
| Density | 1.47g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C23H23N5O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of MeleagrinMeleagrin is a roquefortine C-derived alkaloid produced by fungi of the genus Penicillium and has antimicrobial and anti-proliferative activities. Meleagrin is a class of FabI inhibitor. Meleagrin is a lead c-Met inhibitory entity useful for the control of c-Met-dependent metastatic and invasive breast malignancies[1][2][3]. |
| Name | meleagrine |
|---|---|
| Synonym | More Synonyms |
| Description | Meleagrin is a roquefortine C-derived alkaloid produced by fungi of the genus Penicillium and has antimicrobial and anti-proliferative activities. Meleagrin is a class of FabI inhibitor. Meleagrin is a lead c-Met inhibitory entity useful for the control of c-Met-dependent metastatic and invasive breast malignancies[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Meleagrin inhibits the growth of the human breast cancer cell lines MDA-MB-231, MDA-468, BT-474, SK BR-3, MCF7 and MCF7-dox[3]. Meleagrin causes a dose-dependent inhibition of HGF-induced cell migration, and invasion of breast cancer cell lines[3]. |
| In Vivo | Meleagrin potently suppresses the invasive triple negative breast tumor cell growth in an orthotopic athymic nude mice model[3]. |
| References |
| Density | 1.47g/cm3 |
|---|---|
| Molecular Formula | C23H23N5O4 |
| Molecular Weight | 433.46000 |
| Exact Mass | 433.17500 |
| PSA | 110.79000 |
| LogP | 2.68160 |
| Index of Refraction | 1.717 |
| InChIKey | JTJJJLSLKZFEPJ-ZAYCRUKZSA-N |
| SMILES | C=CC(C)(C)C12C=C(O)C(=O)N3C(=Cc4cnc[nH]4)C(=O)NC31N(OC)c1ccccc12 |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
|
~% 
Meleagrin CAS#:71751-77-4 |
| Literature: Konda, Yaeko; Onda, Masayuki; Hirano, Atsushi; Omura, Satoshi Chemical & Pharmaceutical Bulletin, 1980 , vol. 28, # 10 p. 2987 - 2993 |
|
~% 
Meleagrin CAS#:71751-77-4 |
| Literature: Konda, Yaeko; Onda, Masayuki; Hirano, Atsushi; Omura, Satoshi Chemical & Pharmaceutical Bulletin, 1980 , vol. 28, # 10 p. 2987 - 2993 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
|
[The biosynthesis of low-molecular-weight nitrogen-containing secondary metabolite-alkaloids by the resident strains of Penicillium chrysogenum and Penicillium expansum isolated on the board of the Mir space station ].
Mikrobiologiia 71(6) , 773-7, (2002) The analysis of the absorption spectra of the low-molecular-weight nitrogen-containing secondary metabolites--alkaloids--of 4 Penicillium chrysogenum strains and 6 Penicillium expansum strains isolate... |
|
|
A single cluster of coregulated genes encodes the biosynthesis of the mycotoxins roquefortine C and meleagrin in Penicillium chrysogenum.
Chem. Biol. 18(11) , 1499-512, (2011) A single gene cluster of Penicillium chrysogenum contains genes involved in the biosynthesis and secretion of the mycotoxins roquefortine C and meleagrin. Five of these genes have been silenced by RNA... |
|
|
Roquefortine/oxaline biosynthesis pathway metabolites in Penicillium ser. Corymbifera: in planta production and implications for competitive fitness.
J. Chem. Ecol. 31(10) , 2373-90, (2005) Three strains of each of the seven taxa comprising the Penicillium series Corymbifera were surveyed by direct injection mass spectrometry (MS) and liquid chromatography-MS for the production of terres... |
| [3E,7aR,12aS,(-)]-7a-(1,1-Dimethyl-2-propenyl)-7a,12-dihydro-6-hydroxy-3-(1H-imidazole-4-ylmethylene)-12-methoxy-1H,5H-imidazo[1',2':1,2]pyrido[2,3-b]indole-2(3H),5-dione |
| 8,9-dehydroneoxaline |
| MELEAGRIN |
| Meleagrine |