Aciclovir sodium
Modify Date: 2025-08-25 11:24:06
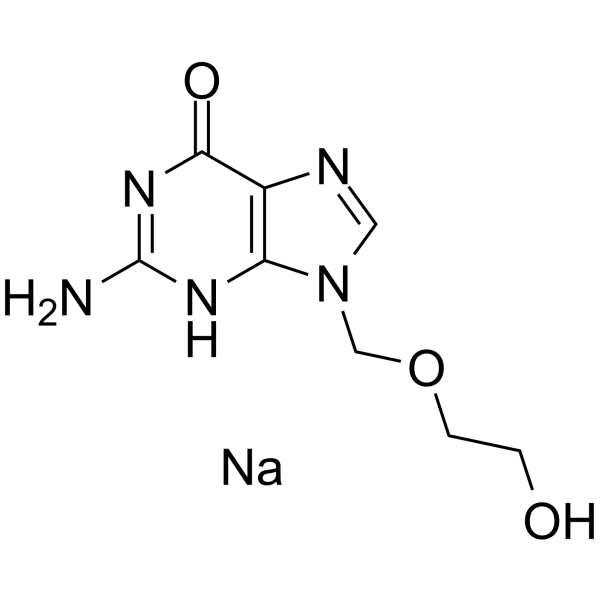
Aciclovir sodium structure
|
Common Name | Aciclovir sodium | ||
|---|---|---|---|---|
| CAS Number | 69657-51-8 | Molecular Weight | 247.18600 | |
| Density | N/A | Boiling Point | 613.1ºC at 760 mmHg | |
| Molecular Formula | C8H10N5NaO3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Aciclovir sodiumAcyclovir (Aciclovir) sodium is a potent, orally active antiviral agent. Acyclovir sodium has antiherpetic activity with IC50 values of 0.85 μM and 0.86 μM for HSV-1 and HSV-2, respectively. Acyclovir sodium induces cell cycle perturbation and apoptosis. Acyclovir sodium prevents bacterial infections during induction therapy for acute leukaemia[1][2][3][4]. |
| Name | Sodium 2-((2-amino-6-oxo-1H-purin-9(6H)-yl)methoxy)ethanolate |
|---|---|
| Synonym | More Synonyms |
| Description | Acyclovir (Aciclovir) sodium is a potent, orally active antiviral agent. Acyclovir sodium has antiherpetic activity with IC50 values of 0.85 μM and 0.86 μM for HSV-1 and HSV-2, respectively. Acyclovir sodium induces cell cycle perturbation and apoptosis. Acyclovir sodium prevents bacterial infections during induction therapy for acute leukaemia[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
HSV-1:0.85 μM (IC50) HSV-2:0.86 μM (IC50) |
| In Vitro | Acyclovir (Aciclovir) sodium (3-100 µM; 24-72 hours; Jurkat, U937, and K562 leukemia cells) reduces cell viability in a dose- and time-dependent[1]. Acyclovir (Aciclovir) sodium (10-100 µM; 24-72 hours; Jurkat cells) blocks DNA synthesis, thereby arresting the cell cycle in G2/M and S phases and increasing the sub-G1 hypodiploid peak in a dose-dependent manner[1]. Acyclovir (Aciclovir) sodium (10-100 µM; 24-72 hours; Jurkat cells) induces apoptosis through activates caspase-3 and presences nuclear DNA fragmentation[1]. Cell Viability Assay[1] Cell Line: Jurkat, U937 and K562 leukemia cells Concentration: 3, 10, 30 and 100 µM Incubation Time: 24, 48 and 72 hours Result: Showed a dose- and time-dependent reduction of cell viability. Apoptosis Analysis[1] Cell Line: Jurkat cells Concentration: 10 and 100 µM Incubation Time: 24, 48 and 72 hours Result: Increased of caspase-3 activity and cleavaged the internucleosomal DNA. Cell Cycle Analysis[1] Cell Line: Jurkat cells Concentration: 10 and 100 µM Incubation Time: 24, 48 and 72 hours Result: Revealed a dose-dependent accumulation of cells in S phase after 24 and 48 h. Showed a dose-dependent increase of the sub-G1 hypodiploid peak after 72 h. |
| In Vivo | Acyclovir (Aciclovir) sodium (20 mg/kg; p.o.; three times daily; for 10 d; BALB/c mice) suppresses the development of skin lesions and results in a dissociation between DTH response and antibody production[3] Animal Model: Specific-pathogen-free BALB/c mice (7-week-old) infected with HSV-1[3] Dosage: 20 mg/kg Administration: Oral administration; three times daily; for 10 days Result: Suppressed the development of skin lesions and resulted in a dissociation between DTH response and antibody production. |
| References |
| Boiling Point | 613.1ºC at 760 mmHg |
|---|---|
| Molecular Formula | C8H10N5NaO3 |
| Molecular Weight | 247.18600 |
| Exact Mass | 247.06800 |
| PSA | 122.14000 |
| LogP | 0.09990 |
| InChIKey | BVOILEATUWMBTI-UHFFFAOYSA-N |
| SMILES | Nc1nc2c(ncn2COCCO)c(=O)[nH]1.[Na] |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Hazard Codes | C |
|---|---|
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| Aciclovir sodium |