Cytarabine hydrochloride
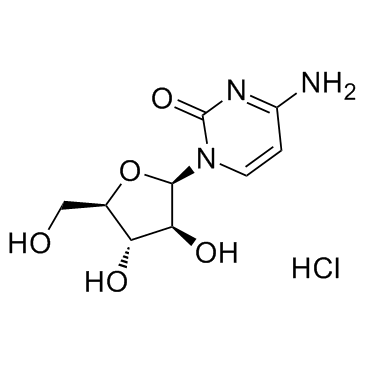
Cytarabine hydrochloride structure
|
Common Name | Cytarabine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 69-74-9 | Molecular Weight | 279.678 | |
| Density | N/A | Boiling Point | 545.7ºC at 760 mmHg | |
| Molecular Formula | C9H14ClN3O5 | Melting Point | 197-198 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 283.8ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
Use of Cytarabine hydrochlorideCytarabine hydrochloride is an antimetabolic agent and DNA synthesis inhibitor with IC50 of 16 nM. |
| Name | Cytarabine hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Cytarabine hydrochloride is an antimetabolic agent and DNA synthesis inhibitor with IC50 of 16 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 16 nM (DNA synthesis) |
| In Vitro | Cytarabine is phosphorylated into a triphosphate form (Ara-CTP) involving deoxycytidine kinase (dCK), which competes with dCTP for incorporation into DNA, and then blocks DNA synthesis by inhibiting the function of DNA and RNA polymerases. Cytarabine displays a higher growth inhibitory activity towards wild-type CCRF-CEM cells compared to other acute myelogenous leukemia (AML) cells with IC50 of 16 nM[1]. Cytarabine apparently induces apoptosis of rat sympathetic neurons at 10 μM, of which 100 μM shows the highest toxicity and kills over 80% of the neurons by 84 hours, involving the release of mitochondrial cytochrome-c and the activation of caspase-3, and the toxicity can be attenuated by p53 knockdown and delayed by bax deletion[2]. |
| In Vivo | Cytarabine (250 mg/kg) also causes placental growth retardation and increases placental trophoblastic cells apoptosis in the placental labyrinth zone of the pregnant Slc:Wistar rats, which increases from 3 hour after the treatment and peaks at 6 hour before returning to control levels at 48 hour, with remarkably enhanced p53 protein, p53 trancriptional target genes such as p21, cyclinG1 and fas and caspase-3 activity[3]. Cytarabine is highly effective against acute leukaemias, which causes the chCytarabineteristic G1/S blockage and synchronization, and increases the survival time for leukaemic Brown Norway rats in a weak dose-related fashion indicating that the use of higher dosages of Cytarabine does not contribute to its antileukaemic effectiveness in man[4]. |
| Kinase Assay | Stock solution of Cytarabine is prepared in absolute ethanol, and serial dilutions of Cytarabine are prepared. CCRF-CEM cells are suspended in RPMI medium supplemented with 10% FBS, 0.1% gentamicin, and 1% sodium pyruvate. The cells are suspended in their respective media to give 10 mL volumes of cell suspension at a final density of 3-6×104 cells/mL. Appropriate volumes of Cytarabine solution are transferred to the cell suspensions, and incubation is continued for 72 hours. The cells are spun down and resuspended in fresh Cytarabine -free medium, and final cell counts are determined. The data are analyzed by sigmoidal curve fitting of the cell count versus Cytarabine concentration, and the results are expressed as the IC50 (Cytarabine concentration that inhibits cell growth to 50% of the control value). |
| Animal Admin | Pregnant rats are injected intraperitoneally (i.p.) with 250 mg/kg of Cytarabine on Day 13 of gestation (GD13). Under the conditions of this experiment, congenital anomalies and growth retardation are detected at a high rate in perinatal fetuses, although the incidence of fetal death is not markedly increased. At 1, 3, 6, 9, 12, 24, and 48 h after the treatment, six dams each are killed by heart puncture under ether anesthesia, and the placentas are collected. As controls, six pregnant rats are injected i.p. with an equivalent volume of PBS on GD13 and killed at the same time point as Cytarabine-treated groups. Of the six dams obtained at each time point, three are used for histopathological analyses and three for reverse transcription-polymerase chain reaction (RT-PCR) analysis. |
| References |
| Boiling Point | 545.7ºC at 760 mmHg |
|---|---|
| Melting Point | 197-198 °C(lit.) |
| Molecular Formula | C9H14ClN3O5 |
| Molecular Weight | 279.678 |
| Flash Point | 283.8ºC |
| Exact Mass | 279.062195 |
| PSA | 130.83000 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317-H319-H341-H361 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R36;R43;R63 |
| Safety Phrases | S26-S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | HA5500000 |
| HS Code | 2942000000 |
|
~94% 
Cytarabine hydr... CAS#:69-74-9 |
| Literature: Boyer, Serge; Erion, Mark D. Patent: US2004/92476 A1, 2004 ; Location in patent: Page/Page column 22 ; |
| HS Code | 2942000000 |
|---|
|
An aberrant sugar modification of BACE1 blocks its lysosomal targeting in Alzheimer's disease.
EMBO Mol. Med. 7(2) , 175-89, (2015) The β-site amyloid precursor protein cleaving enzyme-1 (BACE1), an essential protease for the generation of amyloid-β (Aβ) peptide, is a major drug target for Alzheimer's disease (AD). However, there ... |
|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
|
|
Gingival pain: an unusual side effect of ziprasidone.
BMJ Case Rep. 2013 , doi:10.1136/bcr-2012-007577, (2013) The patient is a 52-year-old man with schizophrenia who developed severe, unremitting gingival pain after his ziprasidone dosage was increased from 80 to 120 mg. His physical examination and laborator... |
| 2(1H)-pyrimidinone, 1-β-D-arabinofuranosyl-3,4-dihydro-4-imino-, hydrochloride (1:1) |
| Cytosine, 1-β-D-arabino-furanosyl-, hydrochloride |
| 1-b-D-Arabinofuranosylcytosine Monohydrochloride |
| Cytosine arabinoside hydrochloride |
| 1-(β-D-Arabinofuranosyl)-4-imino-3,4-dihydropyrimidin-2(1H)-one hydrochloride (1:1) |
| 4-amino-1-[(2R,3S,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2(1H)-one hydrochloride |
| 1-β-D-Arabinofuranosylcytosine hydrochloride |
| Ara-cytidine hydrochloride |
| 4-Amino-1-b-D-arabinofuranosyl-2(1H)-pyrimidinone Monohydrochloride |
| u 19920a |
| 4-Amino-1-(β-D-arabinofuranosyl)pyrimidin-2(1H)-one hydrochloride (1:1) |
| MFCD00012839 |
| EINECS 200-713-9 |
| 4-Amino-1-(β-D-arabinofuranosyl)-2(1H)-pyrimidinone hydrochloride (1:1) |
| 2(1H)-Pyrimidinone, 4-amino-1-β-D-arabinofuranosyl-, hydrochloride (1:1) |
| 4-amino-1-β-D-arabinofuranosylpyrimidin-2(1H)-one hydrochloride |
 CAS#:17676-65-2
CAS#:17676-65-2 CAS#:6742-07-0
CAS#:6742-07-0![[5-(4-acetamido-2-oxo-pyrimidin-1-yl)-3,4-diacetyloxy-oxolan-2-yl]methyl acetate structure](https://image.chemsrc.com/caspic/302/6742-08-1.png) CAS#:6742-08-1
CAS#:6742-08-1
