2-(BOC-Amino)ethanethiol
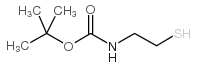
2-(BOC-Amino)ethanethiol structure
|
Common Name | 2-(BOC-Amino)ethanethiol | ||
|---|---|---|---|---|
| CAS Number | 67385-09-5 | Molecular Weight | 177.26 | |
| Density | 1.049 g/mL at 20ºC(lit.) | Boiling Point | 68ºC0.3 mm Hg(lit.) | |
| Molecular Formula | C7H15NO2S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | >230 °F | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 2-(BOC-Amino)ethanethiol2-(Boc-amino)ethanethiol (compound 35) is a bifunctional cross-linker that can be used in the synthesis of bifunctional azobenzene glycoconjugates[1]. |
| Name | 2-(BOC-Amino)ethanethiol |
|---|---|
| Synonym | More Synonyms |
| Description | 2-(Boc-amino)ethanethiol (compound 35) is a bifunctional cross-linker that can be used in the synthesis of bifunctional azobenzene glycoconjugates[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.049 g/mL at 20ºC(lit.) |
|---|---|
| Boiling Point | 68ºC0.3 mm Hg(lit.) |
| Molecular Formula | C7H15NO2S |
| Molecular Weight | 177.26 |
| Flash Point | >230 °F |
| Exact Mass | 177.08200 |
| PSA | 77.13000 |
| LogP | 1.83180 |
| Appearance of Characters | Viscous Liquid or Low Melting Solid | Clear colorless to yellow |
| Index of Refraction | n20/D 1.474(lit.) |
| InChIKey | GSJJCZSHYJNRPN-UHFFFAOYSA-N |
| SMILES | CC(C)(C)OC(=O)NCCS |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2930909090 |
|
~97% 
2-(BOC-Amino)et... CAS#:67385-09-5 |
| Literature: Kim, Young Zu; Kim, Jae Pil Synthetic Communications, 2002 , vol. 32, # 10 p. 1601 - 1605 |
|
~94% 
2-(BOC-Amino)et... CAS#:67385-09-5 |
| Literature: Arisawa, Mieko; Sugata, Chiyoshi; Yamaguchi, Masahiko Tetrahedron Letters, 2005 , vol. 46, # 36 p. 6097 - 6099 |
|
~% 
2-(BOC-Amino)et... CAS#:67385-09-5 |
| Literature: Journal of the Chemical Society, Chemical Communications, , # 24 p. 2571 - 2574 |
|
~% 
2-(BOC-Amino)et... CAS#:67385-09-5 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 19, # 10 p. 2742 - 2746 |
|
~% 
2-(BOC-Amino)et... CAS#:67385-09-5 |
| Literature: Simons, Stoney S.; Pons, Michel; Johnson, David F. Journal of Organic Chemistry, 1980 , vol. 45, # 15 p. 3084 - 3088 |
|
~% 
2-(BOC-Amino)et... CAS#:67385-09-5 |
| Literature: Journal of the American Chemical Society, , vol. 134, # 2 p. 769 - 772 |
| Precursor 6 | |
|---|---|
| DownStream 4 | |
| HS Code | 2930909090 |
|---|---|
| Summary | 2930909090. other organo-sulphur compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Synthesis of Bifunctional Azobenzene Glycoconjugates for Cysteine-Based Photosensitive Cross-Linking with Bioactive Peptides.
Chemistry 21 , 13723-31, (2015) Azobenzene linker molecules can be utilized to control peptide/protein function when they are ligated to appropriately spaced amino acid side chains of the peptide. This is because the photochemical E... |
|
|
Rapid and simple preparation of thiol-ene emulsion-templated monoliths and their application as enzymatic microreactors.
Lab Chip 15 , 2162-72, (2015) A novel, rapid and simple method for the preparation of emulsion-templated monoliths in microfluidic channels based on thiol-ene chemistry is presented. The method allows monolith synthesis and anchor... |
|
|
A convenient route to thiol terminated peptides for conjugation and surface functionalization strategies.
Bioconjug. Chem. 6 , 323, (1995) The derivatization of poly(p-(chloromethyl)styrene-co-divinylbenzene) (Merrifield resin) with N-(tert-butoxycarbonyl)-2-aminoethanethiol is presented as a convenient route for the generation of thiol ... |
| tert-butyl N-(2-sulfanylethyl)carbamate |


![Ethanethioic acid, S-[2-[[(1,1-dimethylethoxy)carbonyl]amino]ethyl] ester (9CI) structure](https://image.chemsrc.com/caspic/002/114326-10-2.png)
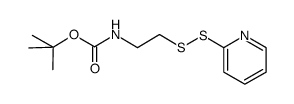 CAS#:535943-48-7
CAS#:535943-48-7![Carbamic acid, [2-(methylthio)ethyl]-, 1,1-dimethylethyl ester (9CI) structure](https://image.chemsrc.com/caspic/193/174360-08-8.png) CAS#:174360-08-8
CAS#:174360-08-8![tert-butyl N-[2-(2-hydroxyethylsulfanyl)ethyl]carbamate structure](https://image.chemsrc.com/caspic/161/75937-17-6.png) CAS#:75937-17-6
CAS#:75937-17-6