| Structure | Name/CAS No. | Articles |
|---|---|---|
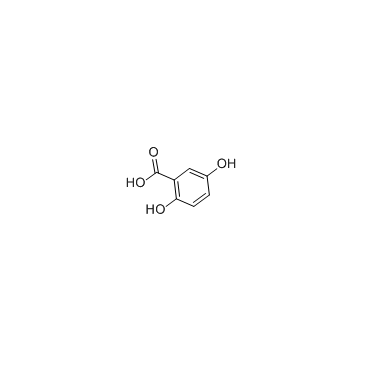 |
Gentisic acid
CAS:490-79-9 |
|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
 |
N,N-Dimethylformamide
CAS:68-12-2 |
|
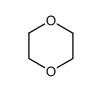 |
1,4-Dioxane
CAS:123-91-1 |
|
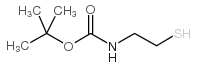 |
2-(BOC-Amino)ethanethiol
CAS:67385-09-5 |
|
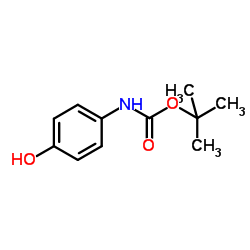 |
tert-Butyl (4-hydroxyphenyl)carbamate
CAS:54840-15-2 |