| Structure | Name/CAS No. | Articles |
|---|---|---|
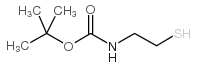 |
2-(BOC-Amino)ethanethiol
CAS:67385-09-5 |
|
 |
DI-TERT-BUTYL (DISULFANEDIYLBIS(ETHANE-2,1-DIYL))DICARBAMATE
CAS:67385-10-8 |