2',5'-Dideoxyadenosine
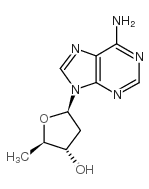
2',5'-Dideoxyadenosine structure
|
Common Name | 2',5'-Dideoxyadenosine | ||
|---|---|---|---|---|
| CAS Number | 6698-26-6 | Molecular Weight | 235.24300 | |
| Density | 1.77 g/cm3 | Boiling Point | 547ºC at 760 mmHg | |
| Molecular Formula | C10H13N5O2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 284.6ºC | |
Use of 2',5'-Dideoxyadenosine2',5'-Dideoxyadenosine is a potent and non-competitive adenylyl cyclase inhibitor via binding the P-site with an IC50 of 3 µM . 2',5'-Dideoxyadenosine is a nucleoside analog and exerts a potent antiadrenergic action in heart[1][2]. |
| Name | 2′,5′-Dideoxyadenosine |
|---|---|
| Synonym | More Synonyms |
| Description | 2',5'-Dideoxyadenosine is a potent and non-competitive adenylyl cyclase inhibitor via binding the P-site with an IC50 of 3 µM . 2',5'-Dideoxyadenosine is a nucleoside analog and exerts a potent antiadrenergic action in heart[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 3 µM (adenylyl cyclase)[1] |
| In Vitro | 2',5'-Dideoxyadenosine (10 μM, 30 min) reduces cAMP production and blocks the phosphorylation of GluA1 at Ser845 induced by carbachol (CCh)[3]. 2',5'-Dideoxyadenosine (10 μM, 30 min) blocks CCh-induced increase of phosphorylation of Akt and attenuates CCh-induced phosphorylation of Ser2448[3]. 2',5'-Dideoxyadenosine (20-150 mM), like adenosine, dependently and reversibly inhibits the positive inotropic and chronotropic effect of beta-adrenergic stimulation with isoproterenol (8-54 pmol) up to 70% and 50%, respectively[2]. Western Blot Analysis[3] Cell Line: Primary hippocampal neurons Concentration: 10 μM Incubation Time: 30 min Result: Reduced cAMP production and blocked the phosphorylation of GluA1 at Ser845 induced by carbachol (CCh). |
| In Vivo | 2',5'-Dideoxyadenosine (0.1 mg/kg; IP; 15 min pre-treated) fully inhibits the diuretic, natriuretic and K+ and Cl- sparing effect of Fr•EtOAc in rats[4]. Animal Model: Male Wistar rats (3-4 months old)[3] Dosage: 0.1 mg/kg Administration: IP; 15 min pre-treated Result: Fully inhibited the diuretic, natriuretic and K+ and Cl- sparing effect of Fr•EtOAc in rats. |
| References |
| Density | 1.77 g/cm3 |
|---|---|
| Boiling Point | 547ºC at 760 mmHg |
| Molecular Formula | C10H13N5O2 |
| Molecular Weight | 235.24300 |
| Flash Point | 284.6ºC |
| Exact Mass | 235.10700 |
| PSA | 99.08000 |
| LogP | 0.65800 |
| Index of Refraction | 1.825 |
| Storage condition | -20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| HS Code | 2934999090 |
|
~72% 
2',5'-Dideoxyad... CAS#:6698-26-6 |
| Literature: Desaubry; Shoshani; Johnson Nucleosides and Nucleotides, 1995 , vol. 14, # 6 p. 1453 - 1460 |
|
~% 
2',5'-Dideoxyad... CAS#:6698-26-6 |
| Literature: Nucleosides and Nucleotides, , vol. 14, # 6 p. 1453 - 1460 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Involvement of cAMP in nerve growth factor-triggered p35/Cdk5 activation and differentiation in PC12 cells.
Am. J. Physiol. Cell Physiol. 299 , C516-27, (2010) The signaling mechanisms underlying cell differentiation have been extensively studied with the use of rat PC12 cells as a model system. Nerve growth factor (NGF) is a trophic factor inducing PC12 cel... |
|
|
A new site and mechanism of action for the widely used adenylate cyclase inhibitor SQ22,536.
Mol. Pharmacol. 83(1) , 95-105, (2013) We evaluated the efficacy, potency, and selectivity of the three most commonly used adenylate cyclase (AC) inhibitors in a battery of cell lines constructed to study signaling via three discrete cAMP ... |
|
|
Identification of 17,20β,21-trihydroxy-4-pregnen-3-one (20β-S) receptor binding and membrane progestin receptor alpha on southern flounder sperm (Paralichthys lethostigma) and their likely role in 20β-S stimulation of sperm hypermotility.
Gen. Comp. Endocrinol. 170(3) , 629-39, (2011) The existence of direct progestin actions on teleost sperm to stimulate hypermotility is not widely acknowledged because it has only been demonstrated in members of the family Sciaenidae. In the prese... |
| 2',5'-dideoxyadenosine |

 CAS#:36792-88-8
CAS#:36792-88-8 CAS#:73-24-5
CAS#:73-24-5