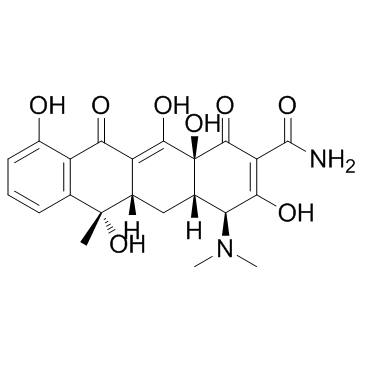
Tetracycline hydrochloride
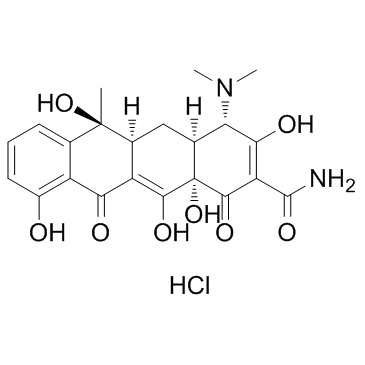
Tetracycline hydrochloride structure
|
Common Name | Tetracycline hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 64-75-5 | Molecular Weight | 480.896 | |
| Density | N/A | Boiling Point | 799.4ºC at 760 mmHg | |
| Molecular Formula | C22H25ClN2O8 | Melting Point | 220-223 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 437.3ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Tetracycline hydrochlorideTetracycline hydrochloride is a broad-spectrum antibiotic used to treat a wide range of infections. |
| Name | Tetracycline hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Tetracycline hydrochloride is a broad-spectrum antibiotic used to treat a wide range of infections. |
|---|---|
| Related Catalog | |
| In Vitro | Tetracyclines are broad-spectrum agents, exhibiting activity against a wide range of gram-positive and gram-negative bacteria, atypical organisms such as chlamydiae, mycoplasmas, and rickettsiae, and protozoan parasites. Tetracyclines inhibit bacterial protein synthesis by preventing the association of aminoacyl-tRNA with the bacterial ribosome. Tetracyclines traverse the outer membrane of gram-negative enteric bacteria through the OmpF and OmpC porin channels, as positively charged cation (probably magnesium)-tetracycline coordination complexes [1]. |
| In Vivo | The tetracyclines have applications for the treatment of infections in poultry, cattle, sheep, and swine. In some cases, e.g., for therapeutic treatment of large numbers of poultry reared on commercial farms, the antibiotics are added directly to feed or water or can be administered in aerosols. Tetracyclines could be used as growth promotion or growth enhancement. Tetracyclines are used in aquaculture to control infections in salmon, catfish, and lobsters[2]. |
| References |
| Boiling Point | 799.4ºC at 760 mmHg |
|---|---|
| Melting Point | 220-223 °C(lit.) |
| Molecular Formula | C22H25ClN2O8 |
| Molecular Weight | 480.896 |
| Flash Point | 437.3ºC |
| Exact Mass | 480.129944 |
| PSA | 181.62000 |
| LogP | 1.28790 |
| Vapour Pressure | 5.82E-27mmHg at 25°C |
| Index of Refraction | -253 ° (C=0.5, 0.1mol/L HCl) |
| Water Solubility | 50 g/L |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | QI9100000 |
| HS Code | 29413000 |
|
~% 
Tetracycline hy... CAS#:64-75-5 |
| Literature: WO2005/112945 A2, ; Page/Page column 106-107 ; |
| Precursor 1 | |
|---|---|
| DownStream 1 | |
| HS Code | 29413000 |
|---|
|
Selective growth-inhibitory effect of 8-hydroxyquinoline towards Clostridium difficile and Bifidobacterium longum subsp. longum in co-culture analysed by flow cytometry.
J. Med. Microbiol. 63(Pt 12) , 1663-9, (2014) The major risk factor for Clostridium difficile infection (CDI) is the use of antibiotics owing to the disruption of the equilibrium of the host gut microbiota. To preserve the beneficial resident pro... |
|
|
Pre-microRNA binding aminoglycosides and antitumor drugs as inhibitors of Dicer catalyzed microRNA processing.
Bioorg. Med. Chem. Lett. 22 , 1709-11, (2012) Over-expressions of miRNAs are being increasingly linked with many diseases including different types of cancer. In this study, the role of some known small molecular therapeutics has been investigate... |
|
|
Development of a Method for the Analysis of Multiclass Antibiotic Residues in Milk Using QuEChERS and Liquid Chromatography-Tandem Mass Spectrometry.
Foodborne Pathog. Dis. 12 , 693-703, (2015) A precise and simplified method of sample preparation for the simultaneous quantification of the antibiotics β-lactam, macrolide, tetracycline, sulfonamide, and quinolone in bovine milk was developed.... |
| achromycin hydrochloride |
| paltet |
| u-5965 |
| EINECS 200-593-8 |
| alatet |
| partrex |
| Tetracycline (hydrochloride) |
| tetracycline HCl |
| teline |
| MFCD00078142 |
| sumycin |
| tet-cy |
| quatrex |
| qidtet |
| unicin |