alpha-Amyrin
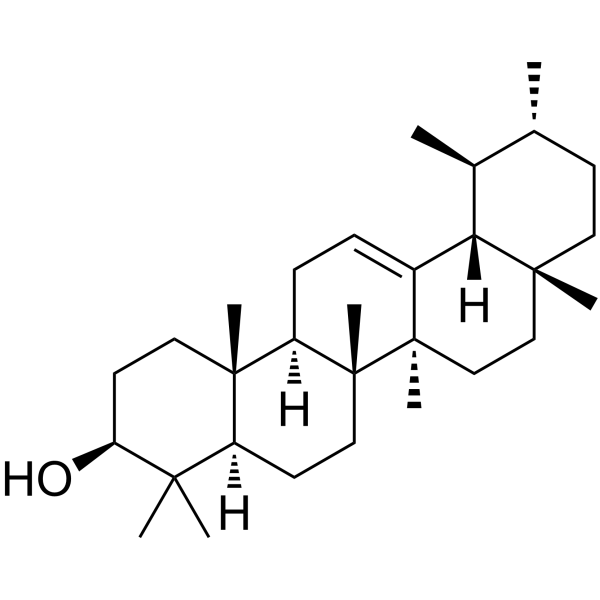
alpha-Amyrin structure
|
Common Name | alpha-Amyrin | ||
|---|---|---|---|---|
| CAS Number | 638-95-9 | Molecular Weight | 426.717 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 493.8±45.0 °C at 760 mmHg | |
| Molecular Formula | C30H50O | Melting Point | 178-183°C | |
| MSDS | Chinese USA | Flash Point | 218.6±21.0 °C | |
Use of alpha-Amyrinα-Amyrin is a pentacyclic triterpenoid. α-Amyrin has long-lasting antinociceptive and anti-inflammatory properties. α-Amyrin can be used for the research of inflammatory[1]. |
| Name | α-amyrin |
|---|---|
| Synonym | More Synonyms |
| Description | α-Amyrin is a pentacyclic triterpenoid. α-Amyrin has long-lasting antinociceptive and anti-inflammatory properties. α-Amyrin can be used for the research of inflammatory[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 493.8±45.0 °C at 760 mmHg |
| Melting Point | 178-183°C |
| Molecular Formula | C30H50O |
| Molecular Weight | 426.717 |
| Flash Point | 218.6±21.0 °C |
| Exact Mass | 426.386169 |
| PSA | 20.23000 |
| LogP | 11.01 |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Index of Refraction | 1.538 |
| Storage condition | 2-8℃ |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
|
β-Amyrin biosynthesis: the critical role of steric volume at C-19 of 2,3-oxidosqualene for its correct folding to generate the pentacyclic scaffold.
Org. Lett. 16(13) , 3548-51, (2014) The effect of the steric volume at C-19 of (3S)-2,3-oxidosqualene 1 on the polycyclization reaction by β-amyrin synthase was examined. The substrate analogs, in which the methyl group at C-19 of 1 was... |
|
|
Comparative analysis of CYP93E proteins for improved microbial synthesis of plant triterpenoids.
Phytochemistry 108 , 47-56, (2014) Cytochrome P450-dependent monooxygenases (P450s) belonging to the CYP93E subfamily catalyze the C-24 oxidation of the triterpene backbone during the biosynthesis of triterpenoid saponins, which are bi... |
|
|
Antitrypanosomal triterpenoid with an ε-lactone E-ring from Salvia urmiensis.
J. Nat. Prod. 76(9) , 1806-9, (2013) A new triterpenoid, urmiensolide (1), was isolated from Salvia urmiensis. The structure was elucidated by a combination of 1D and 2D NMR, HRESIMS, and X-ray crystallographic analyses. The absolute con... |
| α-Amyrenol |
| 4,4,6a,6b,8a,11,12,14b-octamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a |
| 12a,14,14a,14b-icosahydropicen-3-ol |
| a-Amyrenol |
| Urs-12-en-3b-ol |
| VIMINALOL |
| (3S,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-4,4,6a,6b,8a,11,12,14b-Octaméthyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydro-3-picénol |
| AMYRIN,A |
| MFCD00016754 |
| (3S,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-4,4,6a,6b,8a,11,12,14b-Octamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-eicosahydro-picen-3-ol |
| α-Amyrin |
| EINECS 211-352-1 |
| (3S,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-4,4,6a,6b,8a,11,12,14b-Octamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydro-3-picenol |
| Urs-12-en-3β-ol |
| alpha-amyrin |
| 4,4,6a,6b,8a,11,12,14b-octamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicen-3-ol |
| Urs-12-en-3-ol, (3β)- |
| Urs-12-en-3-ol |
| (3β)-Urs-12-en-3-ol |
| a-amyrin |
 CAS#:863-76-3
CAS#:863-76-3 CAS#:545-47-1
CAS#:545-47-1 CAS#:22255-10-3
CAS#:22255-10-3 CAS#:638-96-0
CAS#:638-96-0 CAS#:555-31-7
CAS#:555-31-7 CAS#:107657-17-0
CAS#:107657-17-0 CAS#:141-52-6
CAS#:141-52-6 CAS#:7727-37-9
CAS#:7727-37-9 CAS#:486-34-0
CAS#:486-34-0
