Sodium ethoxide

Sodium ethoxide structure
|
Common Name | Sodium ethoxide | ||
|---|---|---|---|---|
| CAS Number | 141-52-6 | Molecular Weight | 68.050 | |
| Density | 0.868 g/mL at 25 °C | Boiling Point | 91°C | |
| Molecular Formula | C2H5NaO | Melting Point | 260 °C | |
| MSDS | Chinese USA | Flash Point | 22 ºC | |
| Symbol |


GHS02, GHS05 |
Signal Word | Danger | |
| Name | sodium ethoxide |
|---|---|
| Synonym | More Synonyms |
| Density | 0.868 g/mL at 25 °C |
|---|---|
| Boiling Point | 91°C |
| Melting Point | 260 °C |
| Molecular Formula | C2H5NaO |
| Molecular Weight | 68.050 |
| Flash Point | 22 ºC |
| Exact Mass | 68.023811 |
| PSA | 23.06000 |
| LogP | 0.43680 |
| Vapour density | 1.6 (vs air) |
| Vapour Pressure | <0.1 mm Hg ( 20 °C) |
| Index of Refraction | n20/D 1.386 |
| InChIKey | QDRKDTQENPPHOJ-UHFFFAOYSA-N |
| SMILES | CC[O-].[Na+] |
| Storage condition | Store at R.T. |
| Stability | Reacts violently with acids, water. Incompatible with chlorinated solvents, moisture. Absorbs carbon dioxide from the air. Highly flammable. |
| Water Solubility | Miscible |
| Symbol |


GHS02, GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H228-H251-H314 |
| Supplemental HS | Reacts violently with water. |
| Precautionary Statements | P210-P235 + P410-P280-P303 + P361 + P353-P304 + P340 + P310-P305 + P351 + P338 |
| Hazard Codes | C:Corrosive |
| Risk Phrases | R11;R14;R34 |
| Safety Phrases | S8-S16-S26-S43-S45-S36/37/39 |
| RIDADR | UN 3274 3/PG 2 |
| WGK Germany | 1 |
| Packaging Group | II |
| Hazard Class | 4.2 |
| HS Code | 29051900 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 29051900 |
|---|
|
Chemical synthesis and characterization of palm oil-based difatty acyl thiourea.
J. Oleo Sci. 59(5) , 229-33, (2010) Difatty acyl thiourea (DFAT), which has biological activities as antibiotics and antifungal, has been synthesized from palm oil and thiourea using sodium ethoxide as catalyst. Ethyl fatty ester (EFE) ... |
|
|
Bioactivation mechanisms of haloalkene cysteine S-conjugates modeled by gas-phase, ion-molecule reactions.
Chem. Res. Toxicol. 13(7) , 610-5, (2000) Glutathione conjugate formation plays important roles in the detoxification and bioactivation of xenobiotics. A range of nephrotoxic haloalkenes undergo bioactivation that involves glutathione and cys... |
|
|
Fluorometric variable-temperature kinetics investigations of the transesterification reaction of procaine with aliphatic alcohols.
J. Pharm. Pharm. Sci. 7(1) , 88-91, (2004) Variable-Temperature Kinetics has been used to obtain the rate constants of the reaction at various temperatures during one kinetic run.Pseudo-first-order rate constants for the transesterification of... |
| Sodium ethoxide |
| Solid Sodium Ethoxide |
| EINECS 205-487-5 |
| Natriumethanolat |
| SODIUM ETHYLATE |
| sodium methoxide |
| causticalcohol |
| ethoxysodium |
| MFCD00012417 |
| Sodium ethaneolate |
| Sodiumethoxide |
| sodium etoxide |
| SODIUM ETHANOLATE |
| potassium etoxide |
| NE-21 |
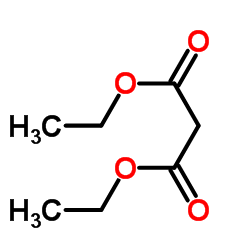 CAS#:105-53-3
CAS#:105-53-3 CAS#:64-17-5
CAS#:64-17-5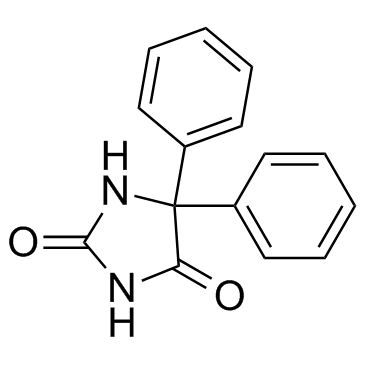 CAS#:57-41-0
CAS#:57-41-0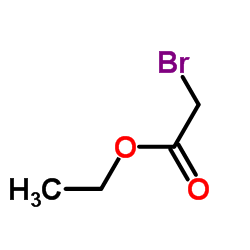 CAS#:105-36-2
CAS#:105-36-2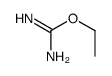 CAS#:28464-55-3
CAS#:28464-55-3![3-Pyridinecarboxylic acid, 6-[(acetyloxy)methyl]-, ethyl ester Structure](https://image.chemsrc.com/caspic/091/91134-19-9.png) CAS#:91134-19-9
CAS#:91134-19-9 CAS#:20737-48-8
CAS#:20737-48-8 CAS#:40545-69-5
CAS#:40545-69-5 CAS#:57999-07-2
CAS#:57999-07-2 CAS#:10475-63-5
CAS#:10475-63-5![2-[diethoxyphosphoryl(phenyl)methoxy]phenol structure](https://image.chemsrc.com/caspic/107/110607-04-0.png) CAS#:110607-04-0
CAS#:110607-04-0 CAS#:119975-32-5
CAS#:119975-32-5 CAS#:111854-29-6
CAS#:111854-29-6 CAS#:74-89-5
CAS#:74-89-5 CAS#:1114-91-6
CAS#:1114-91-6![6-[2-ethoxy-1-(hydroxyamino)ethylidene]cyclohexa-2,4-dien-1-one structure](https://image.chemsrc.com/caspic/155/111249-30-0.png) CAS#:111249-30-0
CAS#:111249-30-0 CAS#:1070-81-1
CAS#:1070-81-1 CAS#:594-27-4
CAS#:594-27-4 CAS#:10602-27-4
CAS#:10602-27-4
