AS-041164
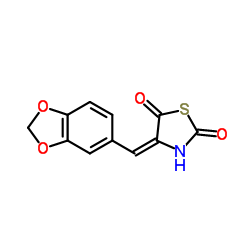
AS-041164 structure
|
Common Name | AS-041164 | ||
|---|---|---|---|---|
| CAS Number | 6318-41-8 | Molecular Weight | 249.243 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C11H7NO4S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of AS-041164AS-041164 is a potent, selective and orally active PI3Kγ isoform inhibitor with an IC50 of 70 nM. AS-041164 shows less activity against PI3Kα, PI3Kβ, and PI3Kδ (IC50s of 240 nM, 1.45 μM, and 1.70 μM, respectively). AS-041164 has anti-inflammatory effects[1]. |
| Name | 5-(1,3-benzodioxol-5-ylmethylidene)-1,3-thiazolidine-2,4-dione |
|---|---|
| Synonym | More Synonyms |
| Description | AS-041164 is a potent, selective and orally active PI3Kγ isoform inhibitor with an IC50 of 70 nM. AS-041164 shows less activity against PI3Kα, PI3Kβ, and PI3Kδ (IC50s of 240 nM, 1.45 μM, and 1.70 μM, respectively). AS-041164 has anti-inflammatory effects[1]. |
|---|---|
| Related Catalog | |
| Target |
PI3Kγ:70 nM (IC50) PI3Kα:240 nM (IC50) PI3Kβ:1.4 μM (IC50) PI3Kδ:1.7 μM (IC50) |
| In Vivo | AS-041164 (10-100 mg/kg; oral administration; once) treatment results in the reduction of inflammatory swelling in the model of carrageenan-induced paw edema[1]. AS-041164 (3-100 mg/kg p.o.) treatment dose-dependently decreases r-h regulated on activation normal T cell expressed and secreted (RANTES)-induced neutrophil recruitment in mice. The ED50 value for AS-041164 is 27.35 mg/kg. AS-041164 blocks RANTES-induced chemotaxis and reduces the level of AKT phosphorylation[1]. Animal Model: Male Wistar rats (100-150 g) injected with carrageenan[1] Dosage: 10 mg/kg, 30 mg/kg, 100 mg/kg Administration: Oral administration; once Result: Induced a significant reduction of paw thickness. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Molecular Formula | C11H7NO4S |
| Molecular Weight | 249.243 |
| Exact Mass | 249.009583 |
| PSA | 89.93000 |
| LogP | 2.46 |
| Index of Refraction | 1.731 |
|
~% 
AS-041164 CAS#:6318-41-8 |
| Literature: Kucera Monatshefte fuer Chemie, 1914 , vol. 35, p. 142 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| 2,5-Thiazolidinedione, 4-(1,3-benzodioxol-5-ylmethylene)-, (4E)- |
| 5-Piperonyliden-2,4-thiazolidindion |
| (4E)-4-(1,3-Benzodioxol-5-ylmethylene)-1,3-thiazolidine-2,5-dione |
| 5-piperonylidene-thiazolidine-2,4-dione |
| 5-Piperonyliden-thiazolidin-2,4-dion |