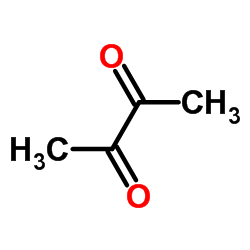
2,3-Butanedione,1,4-dibromo-
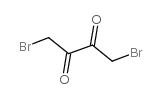
2,3-Butanedione,1,4-dibromo- structure
|
Common Name | 2,3-Butanedione,1,4-dibromo- | ||
|---|---|---|---|---|
| CAS Number | 6305-43-7 | Molecular Weight | 243.88100 | |
| Density | 2.117g/cm3 | Boiling Point | 213ºC at 760 mmHg | |
| Molecular Formula | C4H4Br2O2 | Melting Point | 117-119°C | |
| MSDS | Chinese USA | Flash Point | 86ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 1,4-Dibromo-2,3-Butanedione |
|---|---|
| Synonym | More Synonyms |
| Density | 2.117g/cm3 |
|---|---|
| Boiling Point | 213ºC at 760 mmHg |
| Melting Point | 117-119°C |
| Molecular Formula | C4H4Br2O2 |
| Molecular Weight | 243.88100 |
| Flash Point | 86ºC |
| Exact Mass | 241.85800 |
| PSA | 34.14000 |
| LogP | 0.91440 |
| Index of Refraction | 1.539 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R23/24/25;R34 |
| Safety Phrases | S26-S36/37/39-S37/39-S27 |
| RIDADR | 1759 |
| RTECS | EK2850000 |
| Packaging Group | III |
| Hazard Class | 8 |
| HS Code | 2914700090 |
|
~63% 
2,3-Butanedione... CAS#:6305-43-7 |
| Literature: Liu, Qing-Xiang; Yao, Zhao-Quan; Zhao, Xiao-Jun; Zhao, Zhi-Xiang; Wang, Xiu-Guang Organometallics, 2013 , vol. 32, # 12 p. 3493 - 3501 |
|
~% 
2,3-Butanedione... CAS#:6305-43-7 |
| Literature: Synthesis, , p. 236 - 253 |
|
~% 
2,3-Butanedione... CAS#:6305-43-7 |
| Literature: Chemische Berichte, , vol. 49, p. 1976 |
|
~%
Detail
|
| Literature: International Journal of Chemical Kinetics, , vol. 32, # 7 p. 408 - 418 |
| HS Code | 2914700090 |
|---|---|
| Summary | HS: 2914700090 halogenated, sulphonated, nitrated or nitrosated derivatives of ketones and quinones, whether or not with other oxygen function Tax rebate rate:9.0% Supervision conditions:none VAT:17.0% MFN tariff:5.5% General tariff:30.0% |
|
Diastereoselective formation of a dicopper(i) helicate with a chiral tetradentate pyridylthiazole ligand.
Chem. Commun. (Camb.) (15) , 1999-2001, (2009) Reaction of a pinene-based pyridylthioamide with 1,4-dibromo-2,3-butanedione in refluxing methanol yielded a new chiral pyridylthiazole ligand L which forms a dinuclear double-stranded helicate with C... |
|
|
Cysteinyl peptides labeled by dibromobutanedione in reaction with rabbit muscle pyruvate kinase.
Protein Sci. 1(5) , 678-87, (1992) The bifunctional reagent 1,4-dibromobutanedione (DBBD) reacts covalently with pyruvate kinase from rabbit muscle to cause inactivation of the enzyme at a rate that is linearly dependent on the reagent... |
| MFCD00000205 |
| 1,4-dibromobutane-2,3-dione |
| EINECS 228-615-1 |