1-MONONITROGLYCERIN
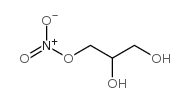
1-MONONITROGLYCERIN structure
|
Common Name | 1-MONONITROGLYCERIN | ||
|---|---|---|---|---|
| CAS Number | 624-43-1 | Molecular Weight | 137.09100 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C3H7NO5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 35.6 °F | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
| Name | 2,3-dihydroxypropyl nitrate |
|---|---|
| Synonym | More Synonyms |
| Molecular Formula | C3H7NO5 |
|---|---|
| Molecular Weight | 137.09100 |
| Exact Mass | 137.03200 |
| PSA | 95.51000 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302 + H312 + H332-H319 |
| Precautionary Statements | P210-P261-P302 + P352 + P312-P304 + P340 + P312-P337 + P313-P403 + P235 |
| RIDADR | UN 1648 3 / PGII |
| Flash Point(F) | 35.6 °F |
| Flash Point(C) | 2 °C |
| Precursor 10 | |
|---|---|
| DownStream 2 | |
|
Studies on the biphasic relaxant curve of glyceryl trinitrate in rat aorta: role of GTN metabolites.
Clin. Exp. Pharmacol. Physiol. 16(11) , 829-35, (1989) 1. The present study has examined the possibility that one or more metabolites of glyceryl trinitrate (GTN) (i.e. glyceryl-1,2- and -1,3-dinitrate and glyceryl-1- and -2-mononitrate) may be responsibl... |
|
|
Guanylate cyclase activation by organic nitrates is not mediated via nitrite.
J. Mol. Cell. Cardiol. 20(5) , 389-96, (1988) Nitrovasodilators relax vascular smooth muscle by stimulating guanylate cyclase. Ignarro et al. (1981) proposed a mechanistic scheme according to which organic nitrates release nitrite in the presence... |
|
|
[Comparative pharmacology of glycerol-1-nitrate and glyceryl trinitrate in various species].
Arzneimittelforschung 36(5) , 814-21, (1986) The present studies yield that all 4 metabolites of glyceryl trinitrate (Nitro Mack, GTN) cause the same typical pharmacological effects as the parent substance. Continuous infusion of 4 mg/kg/min of ... |
| 1,2,3-Propanetriol,1-nitrate |
| Glycerol-1-nitrate |
| Glycerol-1-nitrat |
| glyceryl 1-mononitrate |
| Glycerol 1-mononitrate |
| glycerol nitrate |
| propylene glycol 1-nitrate |
| Glycerol-1-mononitrat |
| 1-Mononitroglycerin |
| 1-Mononitroglycerol |
 CAS#:56-81-5
CAS#:56-81-5 CAS#:4704-77-2
CAS#:4704-77-2 CAS#:556-52-5
CAS#:556-52-5 CAS#:16770-74-4
CAS#:16770-74-4 CAS#:55-63-0
CAS#:55-63-0 CAS#:623-87-0
CAS#:623-87-0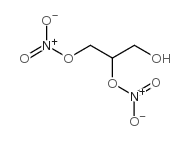 CAS#:621-65-8
CAS#:621-65-8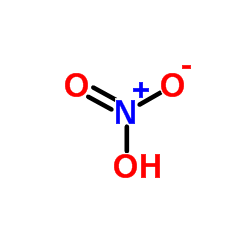 CAS#:7697-37-2
CAS#:7697-37-2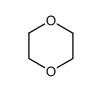 CAS#:123-91-1
CAS#:123-91-1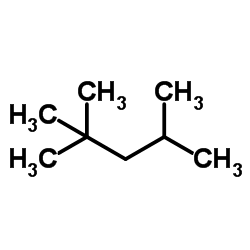 CAS#:540-84-1
CAS#:540-84-1