Tenuazonic acid-(Copper salt)
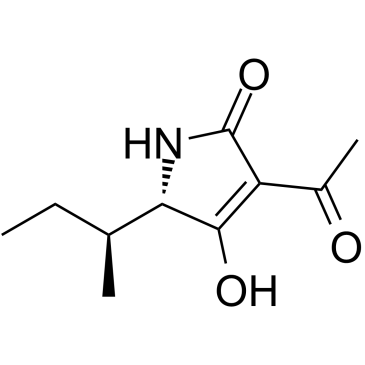
Tenuazonic acid-(Copper salt) structure
|
Common Name | Tenuazonic acid-(Copper salt) | ||
|---|---|---|---|---|
| CAS Number | 610-88-8 | Molecular Weight | 197.231 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 401.1±45.0 °C at 760 mmHg | |
| Molecular Formula | C10H15NO3 | Melting Point | 74.5°C | |
| MSDS | Chinese USA | Flash Point | 196.4±28.7 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of Tenuazonic acid-(Copper salt)Tenuazonic acid, belonging to tetramic acids that are the largest family of natural products, is a putative nonhost-selective mycotoxin isolated from Alternaria alternate[1]. Tenuazonic acid blocks electron transport beyond primary quinone acceptor (QA) by interacting with D1 protein and it is a broad-spectrum and effective photosystem II (PSII) inhibitor[2]. |
| Name | Tenuazonic Acid |
|---|---|
| Synonym | More Synonyms |
| Description | Tenuazonic acid, belonging to tetramic acids that are the largest family of natural products, is a putative nonhost-selective mycotoxin isolated from Alternaria alternate[1]. Tenuazonic acid blocks electron transport beyond primary quinone acceptor (QA) by interacting with D1 protein and it is a broad-spectrum and effective photosystem II (PSII) inhibitor[2]. |
|---|---|
| Related Catalog | |
| Target |
PSII[2] |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 401.1±45.0 °C at 760 mmHg |
| Melting Point | 74.5°C |
| Molecular Formula | C10H15NO3 |
| Molecular Weight | 197.231 |
| Flash Point | 196.4±28.7 °C |
| Exact Mass | 197.105194 |
| PSA | 66.40000 |
| LogP | 0.81 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.519 |
| InChIKey | CEIZFXOZIQNICU-XNCJUZBTSA-N |
| SMILES | CCC(C)C1NC(=O)C(C(C)=O)=C1O |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | UY7425000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
|
~% 
Tenuazonic acid... CAS#:610-88-8 |
| Literature: Matsuo; Kitaguchi; Takata; Tanaka Chemical and Pharmaceutical Bulletin, 1980 , vol. 28, # 8 p. 2494 - 2502 |
|
~% 
Tenuazonic acid... CAS#:610-88-8 |
| Literature: Poncet, Joel; Jouin, Patrick; Castro, Bertrand; Nicolas, Louisette; Boutar, Mohamed; Gaudemer, Alain Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1990 , p. 611 - 616 |
|
~% 
Tenuazonic acid... CAS#:610-88-8 |
| Literature: Poncet, Joel; Jouin, Patrick; Castro, Bertrand; Nicolas, Louisette; Boutar, Mohamed; Gaudemer, Alain Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1990 , p. 611 - 616 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Natural occurrence of four Alternaria mycotoxins in tomato- and citrus-based foods in China.
J. Agric. Food Chem. 63(1) , 343-8, (2015) A total of 70 tomato-based and 86 citrus-based products collected in China were analyzed for alternariol, alternariol monomethyl ether, tentoxin, and tenuazonic acid by ultraperformance liquid chromat... |
|
|
Evaluation of analogs of reutericyclin as prospective candidates for treatment of staphylococcal skin infections.
Antimicrob. Agents Chemother. 53 , 4028-31, (2009) The potential for reutericyclin derivatives to be used as topical antibiotics to treat staphylococcal skin infections was investigated. All reutericyclins inhibited the growth of clinical isolates of ... |
|
|
Effects of nisin and reutericyclin on resistance of endospores of Clostridium spp. to heat and high pressure.
Food Microbiol. 34(1) , 46-51, (2013) The effects of high pressure, temperature, and antimicrobial compounds on endospores of Clostridium spp. were examined. Minimal inhibitory concentrations (MIC) of nisin and reutericyclin were determin... |
| 3-acetyl-5-(butan-2-yl)-4-hydroxy-1,5-dihydro-2H-pyrrol-2-one |
| 3-Acetyl-5-sec-butyl-4-hydroxy-1,5-dihydro-2H-pyrrol-2-one |
| 4-acetyl-2-butan-2-yl-3-hydroxy-1,2-dihydropyrrol-5-one |
| 2H-Pyrrol-2-one, 3-acetyl-1,5-dihydro-4-hydroxy-5-(1-methylpropyl)- |