Zoxazolamine
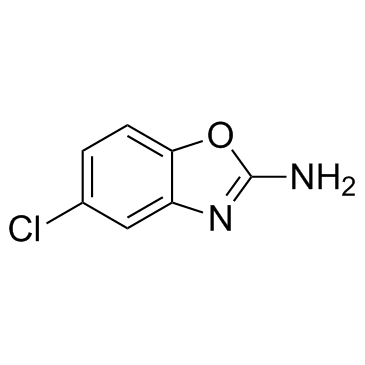
Zoxazolamine structure
|
Common Name | Zoxazolamine | ||
|---|---|---|---|---|
| CAS Number | 61-80-3 | Molecular Weight | 168.58000 | |
| Density | 1.481 g/cm3 | Boiling Point | 316.8ºC at 760 mmHg | |
| Molecular Formula | C7H5ClN2O | Melting Point | 181-184 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 145.4ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of ZoxazolamineZoxazolamine is widely used for a pharmacologic test that serves as a convenient indicator of changes in cytochrome P-450 activity in rodents. |
| Name | 2-Amino-5-chlorobenzoxazole |
|---|---|
| Synonym | More Synonyms |
| Description | Zoxazolamine is widely used for a pharmacologic test that serves as a convenient indicator of changes in cytochrome P-450 activity in rodents. |
|---|---|
| Related Catalog | |
| In Vivo | Zoxazolamine is widely used for a pharmacologic test that serves as a convenient indicator of changes in cytochrome P-450 activity in rodents. The time-averaged serum clearance determined from tested concentrations data is 7.22±1.01 mL/min/kg for Zoxazolamine. Infusion of Zoxazolamine to righting reflex (LRR) at three different rates shows increasing Zoxazolamine concentration in serum and brain at the onset of the effect with increasing infusion rates. When the Zoxazolamine infusion is continued for 5 min beyond the onset of LRR, the concentrations of Zoxazolamine in the cerebrospinal fluid (CSF) at the offset of LRR are essentially identical to the onset concentrations[1]. |
| Animal Admin | Inbred male Lewis rats are used in this study. The animals are allowed 7 to 12 days to adjust to the local animal facilities and to recover possible stress incurred during transport. To compare drug concentrations in serum at the onset and offset of loss of the righting reflex, Zoxazolamine is infused into the femoral vein at a rate of 0.51 mg/min for 5 min beyond the onset of loss of the righting reflex. The infusion solution consists of 10 mg Zoxazolamine in 1 mL normal saline. Then, blood samples from the jugular vein are obtained from four rates at the onset and offset of loss of the righting reflex, using a procedure that yields mainstream blood[1]. |
| References |
| Density | 1.481 g/cm3 |
|---|---|
| Boiling Point | 316.8ºC at 760 mmHg |
| Melting Point | 181-184 °C(lit.) |
| Molecular Formula | C7H5ClN2O |
| Molecular Weight | 168.58000 |
| Flash Point | 145.4ºC |
| Exact Mass | 168.00900 |
| PSA | 52.05000 |
| LogP | 2.64460 |
| Index of Refraction | 1.692 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H312-H315-H319-H332-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R20/21/22;R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DM4550000 |
| HS Code | 2934999090 |
|
~% 
Zoxazolamine CAS#:61-80-3 |
| Literature: US2003/9034 A1, ; US2003/109714 A1, ; |
|
~35% 
Zoxazolamine CAS#:61-80-3 |
| Literature: Kloeckner, Ulrich; Weckenmann, Nicole M.; Nachtsheim, Boris J. Synlett, 2012 , # 1 art. no. D23611ST, p. 97 - 100 |
|
~% 
Zoxazolamine CAS#:61-80-3 |
| Literature: Journal of the American Chemical Society, , vol. 75, p. 2770 |
|
~% 
Zoxazolamine CAS#:61-80-3 |
| Literature: Journal of the American Chemical Society, , vol. 75, p. 2770 |
|
~% 
Zoxazolamine CAS#:61-80-3 |
| Literature: Journal of the American Chemical Society, , vol. 75, p. 2770 |
|
~% 
Zoxazolamine CAS#:61-80-3 |
| Literature: US2780633 , ; |
|
~% 
Zoxazolamine CAS#:61-80-3 |
| Literature: US2890985 , ; |
|
~% 
Zoxazolamine CAS#:61-80-3 |
| Literature: Annali di Chimica (Rome, Italy), , vol. 48, p. 657,660 |
|
~% 
Zoxazolamine CAS#:61-80-3 |
| Literature: Gazzetta Chimica Italiana, , vol. 89, p. 1009,1013 |
| Precursor 10 | |
|---|---|
| DownStream 8 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Isolation, structure elucidation and in vivo hepatoprotective potential of trans-tetracos-15-enoic acid from Indigofera tinctoria Linn.
Phytother Res. 20(10) , 831-9, (2006) The bioassay guided fractionation of the dried aerial part of Indigofera tinctoria Linn. led to the identification of an active fraction labelled as indigotin. On further chemical analysis, a compound... |
|
|
Pharmacological activation of cloned intermediate- and small-conductance Ca(2+)-activated K(+) channels.
Am. J. Physiol. 278 , C570-C581, (2000) We previously characterized 1-ethyl-2-benzimidazolinone (1-EBIO), as well as the clinically useful benzoxazoles, chlorzoxazone (CZ), and zoxazolamine (ZOX), as pharmacological activators of the interm... |
|
|
Stimulation of Cl(-) secretion by chlorzoxazone.
J. Pharmacol. Exp. Ther. 292 , 778-787, (2000) We previously demonstrated that 1-ethyl-2-benzimidazolone (1-EBIO) directly activates basolateral membrane calcium-activated K(+) channels (K(Ca)), thereby stimulating Cl(-) secretion across several e... |
| EINECS 200-519-4 |
| MFCD00005770 |
| 5-chloro-1,3-benzoxazol-2-amine |
| Zoxazolamine |

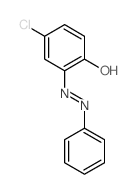
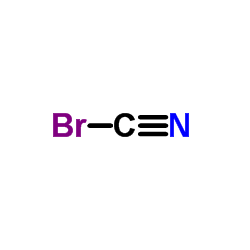
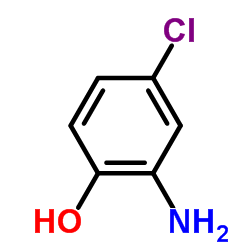

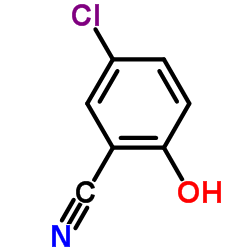
 CAS#:13988-20-0
CAS#:13988-20-0 CAS#:5285-41-6
CAS#:5285-41-6 CAS#:5790-90-9
CAS#:5790-90-9 CAS#:64037-08-7
CAS#:64037-08-7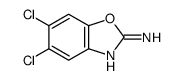 CAS#:64037-12-3
CAS#:64037-12-3 CAS#:64037-19-0
CAS#:64037-19-0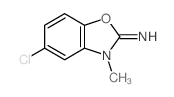 CAS#:64037-23-6
CAS#:64037-23-6
