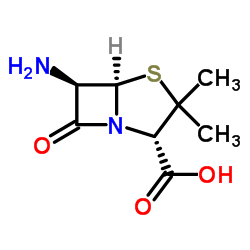
Cloxacillin
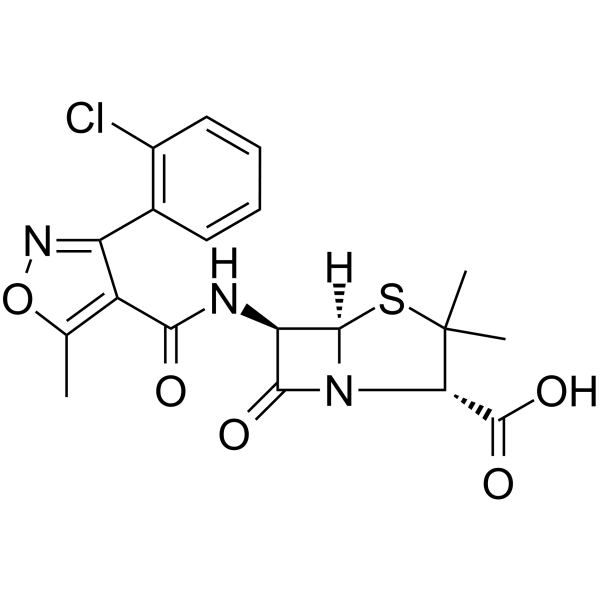
Cloxacillin structure
|
Common Name | Cloxacillin | ||
|---|---|---|---|---|
| CAS Number | 61-72-3 | Molecular Weight | 435.881 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 689.7±55.0 °C at 760 mmHg | |
| Molecular Formula | C19H18ClN3O5S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 370.9±31.5 °C | |
Use of CloxacillinCloxacillin is an orally active antibacterial agent and β-lactamase inhibitor with an IC50 of 0.04 µM. Cloxacillin can suppress the S. aureus-induced inflammatory response by inhibiting the activation of MAPKs, NF-кB and NLRP3-related proteins[1][2][3]. |
| Name | cloxacillin |
|---|---|
| Synonym | More Synonyms |
| Description | Cloxacillin is an orally active antibacterial agent and β-lactamase inhibitor with an IC50 of 0.04 µM. Cloxacillin can suppress the S. aureus-induced inflammatory response by inhibiting the activation of MAPKs, NF-кB and NLRP3-related proteins[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.04 µM (β-lactamase from wild type A.caviae), 0.3 µM (β-lactamase from mutant A.caviae)[3]. |
| In Vitro | Cloxacillin (0-2048 µg/mL; 20-24 h) shows good antibacterial activity for S. aureus 8325-4 and DU1090 with MIC values both of 0.125 µg/mL[1]. Cloxacillin (0.015625 μg/mL; 6 h) inhibits the hemolytic activity of Hlα in vitro, and this inhibition is not only more pronounced when combined with TZ and TZ, but also suppresses the inflammatory response by inhibiting the activation of MAPKs, NF-кB and NLRP3-related proteins[1]. Cell Viability Assay[1] Cell Line: S. aureus 8325-4, S. aureus DU1090 (an Hlα-deleted strain) Concentration: 0-2048 µg/mL Incubation Time: 20-24 h Result: Inhibited S. aureus 8325-4 and DU1090 with MIC values both of 0.125 µg/mL. Western Blot Analysis[1] Cell Line: S. aureus 8325-4 Concentration: 0.015625 μg/mL (combines with Thioridazine (TZ, 0.25 μg/mL) and Tetracycline (TC, 0.03125 μg/mL)). Incubation Time: 6 h Result: Inhibited the expression of Hlα and the inhibition was more pronounced when combined with TZ and TC. Western Blot Analysis[1] Cell Line: RAW264.7 cells (exposes to S. aureus 8325-4/DU1090 or pure Hlα) Concentration: 0.015625 μg/mL (combines with TZ (0.25 μg/mL) and TC (0.03125 μg/mL)). Incubation Time: 6 h Result: Inhibited the activation of MAPKs, NF-кB and NLRP3-related proteins thereby inhibiting the inflammatory response when combined with TC and TZ. |
| In Vivo | Cloxacillin (1.6125 mg/kg; s.c.; 12-h intervals for 72 h) protects mice from S. aureus peritonitis in vivo when combines with Thioridazine and Tetracycline[1]. Cloxacillin (7.5 mg/per; i.p.; twice daily from day 3 for 3 days) develops less severe synovitis and reduces bone erosions when combines with anti-IL-15 antibodies[3]. Animal Model: Female BALB/c mice (6-week-old; peritonitis model)[1]. Dosage: 1.6125 mg/kg (combines with TC (3.125 mg/kg) and TZ (25 mg/kg)) Administration: Subcutaneous injection; 12-h intervals for 72 h. Result: Reduced the degree of inflammatory cell infiltration in the mouse lung tissue and alveolar structures tended to be normal. Significantly reduced the pathological changes in spleen and liver tissue, as well as decreased the CFU counts of S. aureus in the peritoneal cavity. Animal Model: Female wildtype C57BL/6 mice (8-week-old; systemic S. aureus-induced arthritis model) Dosage: 7.5 mg/per (combines with 25 µg/per anti-IL-15 antibodies) Administration: Intraperitoneal injection; twice daily from day 3 (after bacterial inoculation) and stopped at day 6. Result: Showed activities of reducing severe synovitis and bone erosions when combined with anti-IL-15 antibodies. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 689.7±55.0 °C at 760 mmHg |
| Molecular Formula | C19H18ClN3O5S |
| Molecular Weight | 435.881 |
| Flash Point | 370.9±31.5 °C |
| Exact Mass | 435.065582 |
| PSA | 138.04000 |
| LogP | 2.53 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.685 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
|
~% 
Cloxacillin CAS#:61-72-3 |
| Literature: Journal of the Chemical Society, , p. 5838 - 5845 |
|
~% 
Cloxacillin CAS#:61-72-3 |
| Literature: Journal of the Chemical Society, , p. 5838 - 5845 |
|
~% 
Cloxacillin CAS#:61-72-3 |
| Literature: Journal of the Chemical Society, , p. 5838 - 5845 |
|
~% 
Cloxacillin CAS#:61-72-3 |
| Literature: Journal of the Chemical Society, , p. 5838 - 5845 |
|
~% 
Cloxacillin CAS#:61-72-3 |
| Literature: Journal of the Chemical Society, , p. 5838 - 5845 |
| xo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylicacid |
| Cloxacillin (base and/or unspecified salts) |
| (2S,5R,6R)-6-[[[3-(2-Chlorophenyl)-5-methyl-4-isoxazolyl]carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid |
| [5-Methyl-3-(o-chlorophenyl)-4-isoxazolyl]penicillin |
| MFCD00150735 |
| [3-(o-chlorophenyl)-5-methyl-4-isoxazolyl]penicillin |
| methocillins |
| [2S-(2a,5a,6b)]-6-[[[3-(2-Chlorophenyl)-5-methyl-4-isoxazolyl]carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid |
| (2S,5R,6R)-6-({[3-(2-Chlorophenyl)-5-methyl-1,2-oxazol-4-yl]carbonyl}amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| 3-dimethyl-7-oxo-thyl-4-isoxazolecarboxamido) |
| Orbenin Extra Dry Cow |
| brl1621 |
| 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[[3-(2-chlorophenyl)-5-methyl-4-isoxazolyl]carbonyl]amino]-3,3-dimethyl-7-oxo-, (2S,5R,6R)- |
| cloxacillin |
| Syntarpen |
| EINECS 200-514-7 |
| MCIPC |
| 6-<5-Methyl-3-(2-chlor-phenyl)-isoxazolyl-(4)-formamino>-penicillansaeure |