CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
RB6880000
-
CHEMICAL NAME :
-
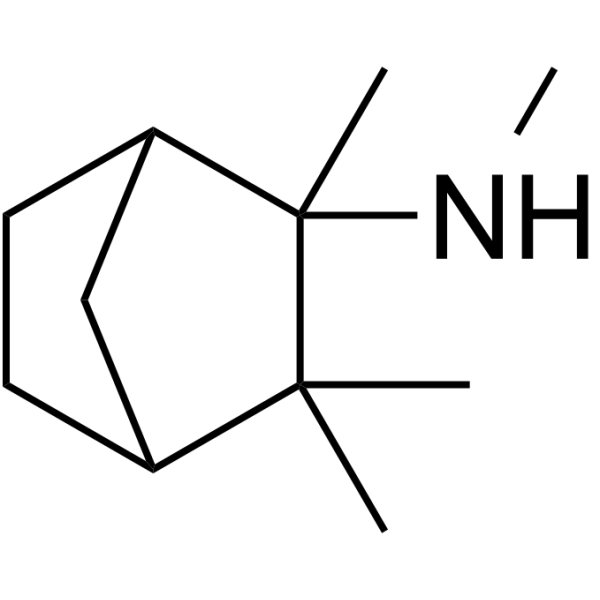
2-Norbornanamine, N,2,3,3-tetramethyl-
-
CAS REGISTRY NUMBER :
-
60-40-2
-
LAST UPDATED :
-
199703
-
DATA ITEMS CITED :
-
4
-
MOLECULAR FORMULA :
-
C11-H21-N
-
MOLECULAR WEIGHT :
-
167.33
-
WISWESSER LINE NOTATION :
-
L55 ATJ CM1 C1 D1 D1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
90 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
BCFAAI Bollettino Chimico Farmaceutico. (Societa Editoriale Farmaceutica, Via Ausonio 12, 20123 Milan, Italy) V.33- 1894- Volume(issue)/page/year: 103,490,1964
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
40 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - sleep
-
REFERENCE :
-
AITEAT Archivum Immunologiae et Therapiae Experimentalis. (Ars Polona, POB 1001, 00-068 Warsaw 1, Poland) V.10- 1962- Volume(issue)/page/year: 10,905,1962
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
37500 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
FATOAO Farmakologiya i Toksikologiya (Moscow). For English translation, see PHTXA6 and RPTOAN. (V/O Mezhdunarodnaya Kniga, 113095 Moscow, USSR) V.2- 1939- Volume(issue)/page/year: 25,163,1962
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
11900 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
USXXAM United States Patent Document. (U.S. Patent Office, Box 9, Washington, DC 20231) Volume(issue)/page/year: #4168308
|