Andrographolide
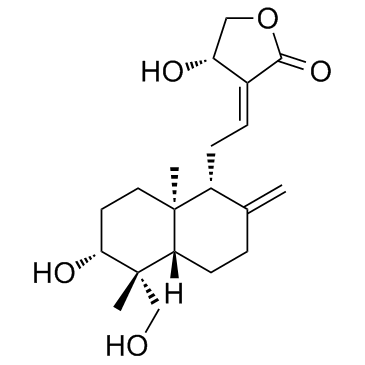
Andrographolide structure
|
Common Name | Andrographolide | ||
|---|---|---|---|---|
| CAS Number | 5508-58-7 | Molecular Weight | 350.449 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 557.3±50.0 °C at 760 mmHg | |
| Molecular Formula | C20H30O5 | Melting Point | 229-232ºC | |
| MSDS | Chinese USA | Flash Point | 195.5±23.6 °C | |
Use of AndrographolideAndrographolide is a NF-κB inhibitor, which inhibits NF-κB activation through covalent modification of a cysteine residue on p50 in endothelial cells without affecting IκBα degradation or p50/p65 nuclear translocation. |
| Name | andrographolide |
|---|---|
| Synonym | More Synonyms |
| Description | Andrographolide is a NF-κB inhibitor, which inhibits NF-κB activation through covalent modification of a cysteine residue on p50 in endothelial cells without affecting IκBα degradation or p50/p65 nuclear translocation. |
|---|---|
| Related Catalog | |
| Target |
p50 |
| In Vitro | Andrographolide (AP) concentration-dependently suppresses receptor activator of nuclear factor kappa B ligand (RANKL)-mediated osteoclast differentiation and bone resorption in vitro and reduces the expression of osteoclast-specific markers. Andrographolide attenuates inflammation by inhibition of TNFα-induced NF-κB activation through covalent modification of reduced Cys62 of p50, without affecting IκBα degradation or p50/p65 nuclear translocation. Andrographolide also inhibits the ERK/MAPK signalling pathway without affecting p38 or JNK signalling. Andrographolide inhibits osteoclast differentiation of RAW 264.7 cells in a concentration-dependent manner. Andrographolide suppresses osteoclast formation in a concentration-dependent manner without any obvious cytotoxic effects, in both BMMs and RAW 264.7 cells. Andrographolide treatment substantially reduces the area of bone resorption. Only approximately 30% of the bone resorption observed in the control group is achieved after treatment with 2.5 μM Andrographolide. Osteoclastic bone resorption is almost completely inhibited after treatment with 10 μM Andrographolide[1]. |
| In Vivo | Treatment with Andrographolide (5 or 30 mg/kg) reduces the extent of bone loss induced by LPS. Moreover, Andrographolide slightly increases the BMD and cortex thickness compared to LPS treatment. Histological examination confirms the protective effects of Andrographolide on LPS-induced bone loss. LPS injection leads to inflammatory bone erosion and increased numbers of TRAP-positive osteoclasts[1]. |
| Kinase Assay | In vitro osteoclastogenesis assays are preformed to examine the effects of Andrographolide on osteoclast differentiation. Bone marrow macrophages (BMM) cells are prepared. Briefly, cells extracted from the femur and tibiae of a 6-week-old C57/BL6 mouse are incubated in complete cell culture media and 30 ng/mL M-CSF in a T-75 cm2 flask for proliferation. When changing the medium, the cells are washed in order to deplete residual stromal cells. After reaching 90% confluence, cells are washed with PBS three times and trypsinized for 30 min to harvest BMMs. Cells adhering to the bottom of the dish are classified as BMMs; these BMMs are plated in 96-well plates at a density of 8×103 cells per well in triplicate and incubated in a humidified incubator containing 5% CO2 at 37°C for 24 h. The cells are then treated with various concentrations of Andrographolide (0, 2.5, 5, or 10 μM) plus M-CSF (30 ng/mL) and RANKL (50 ng/mL). After 5 days, cells are fixed and stained for tartrate-resistant acid phosphatase (TRAP) activity. TRAP-positive multinucleated cells with more than five nuclei are counted as osteoclasts[1]. |
| Cell Assay | Effects of Andrographolide on cell proliferation are determined with a CCK-8. BMMs are plated in 96-well plates at a density of 3×103 cells per well in triplicate. Twenty-four hours later, the cells are treated with increasing concentrations of Andrographolide (0, 2.5, 5, 10 or 20 μM) for 2 days. Next, 10 μL CCK-8 is added to each well, and the plates are then incubated at 37°C for an additional 2 h. The optical density (OD) is then measured with an ELX800 absorbance microplate reader at a wavelength of 450 nm (650 nm reference). The cell viability is calculated[1]. |
| Animal Admin | Mice[1] C57BL/6 mice (8 weeks old) are divided into four groups of seven mice each. Mice are injected i.p. with Andrographolide (5 or 30 mg/kg body weight) or PBS as a control 1 day before injection of LPS (5 μg/g body weight). Andrographolide or PBS is injected intraperitoneally every other day for 8 days. LPS is injected intraperitoneally on days one and four. All mice are killed 8 days after the initial LPS injection, and the left femurs of all animals are scanned with a high-resolution micro-CT at a resolution of 9 μm. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 557.3±50.0 °C at 760 mmHg |
| Melting Point | 229-232ºC |
| Molecular Formula | C20H30O5 |
| Molecular Weight | 350.449 |
| Flash Point | 195.5±23.6 °C |
| Exact Mass | 350.209320 |
| PSA | 86.99000 |
| LogP | 1.62 |
| Vapour Pressure | 0.0±3.4 mmHg at 25°C |
| Index of Refraction | 1.568 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn |
|---|---|
| Risk Phrases | R20/21/22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | LU3490750 |
| HS Code | 29322980 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 29322980 |
|---|
|
Andrographolide prevents high-fat diet-induced obesity in C57BL/6 mice by suppressing the sterol regulatory element-binding protein pathway.
J. Pharmacol. Exp. Ther. 351(2) , 474-83, (2014) Sterol regulatory element-binding proteins (SREBPs) are major transcription factors regulating the expression of genes involved in biosynthesis of cholesterol, fatty acids, and triglycerides. We inves... |
|
|
Bioavailability of andrographolide and protection against carbon tetrachloride-induced oxidative damage in rats.
Toxicol. Appl. Pharmacol. 280(1) , 1-9, (2014) Andrographolide, a bioactive diterpenoid, is identified in Andrographis paniculata. In this study, we investigated the pharmacokinetics and bioavailability of andrographolide in rats and studied wheth... |
|
|
Andrographolide inhibits TNFα-induced ICAM-1 expression via suppression of NADPH oxidase activation and induction of HO-1 and GCLM expression through the PI3K/Akt/Nrf2 and PI3K/Akt/AP-1 pathways in human endothelial cells.
Biochem. Pharmacol. 91(1) , 40-50, (2014) Andrographolide, the major bioactive component of Andrographis paniculata, has been demonstrated to have various biological properties including anti-inflammation, antioxidation, and anti-hepatotoxici... |
| ANDROGRAPHIS |
| Rhizoma Sparganii |
| EINECS 226-852-5 |
| Andrographolid |
| MFCD07778082 |
| ANDRO |
| Andrographalide |
| Andrographolide |
 CAS#:2131-43-3
CAS#:2131-43-3
