4,4-Dimethoxy-2-butanone
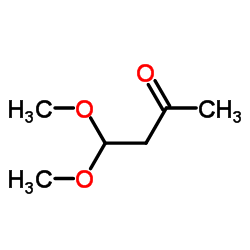
4,4-Dimethoxy-2-butanone structure
|
Common Name | 4,4-Dimethoxy-2-butanone | ||
|---|---|---|---|---|
| CAS Number | 5436-21-5 | Molecular Weight | 132.158 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 172.1±20.0 °C at 760 mmHg | |
| Molecular Formula | C6H12O3 | Melting Point | -82 °C | |
| MSDS | Chinese USA | Flash Point | 49.4±0.0 °C | |
| Symbol |

GHS02 |
Signal Word | Warning | |
Use of 4,4-Dimethoxy-2-butanone4,4-Dimethoxy-2-butanone is an endogenous metabolite. |
| Name | Acetylacetaldehyde dimethyl acetal |
|---|---|
| Synonym | More Synonyms |
| Description | 4,4-Dimethoxy-2-butanone is an endogenous metabolite. |
|---|---|
| Related Catalog |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 172.1±20.0 °C at 760 mmHg |
| Melting Point | -82 °C |
| Molecular Formula | C6H12O3 |
| Molecular Weight | 132.158 |
| Flash Point | 49.4±0.0 °C |
| Exact Mass | 132.078644 |
| PSA | 35.53000 |
| LogP | 0.33 |
| Vapour Pressure | 1.4±0.3 mmHg at 25°C |
| Index of Refraction | 1.399 |
| InChIKey | PJCCSZUMZMCWSX-UHFFFAOYSA-N |
| SMILES | COC(CC(C)=O)OC |
| Storage condition | Flammables area |
| Water Solubility | decomposes |
| Symbol |

GHS02 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H226 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | F |
| Risk Phrases | R10 |
| Safety Phrases | S16 |
| RIDADR | UN 1989 3/PG 3 |
| WGK Germany | 2 |
| RTECS | EL7592500 |
| Packaging Group | III |
| Hazard Class | 3 |
| HS Code | 29145000 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Carbonylation as a key reaction in anaerobic acetone activation by Desulfococcus biacutus.
Appl. Environ. Microbiol. 79(20) , 6228-35, (2013) Acetone is activated by aerobic and nitrate-reducing bacteria via an ATP-dependent carboxylation reaction to form acetoacetate as the first reaction product. In the activation of acetone by sulfate-re... |
|
|
Yamadazyma Farinosa IFO 10896-mediated reduction of 4, 4-dimethoxy-2-butanone as the key-step for the preparation of 1, 3-diols with unsymmetrical substituents. Yamazaki T, et al.
Synth. Commun. 30(16) , 3061-72, (2000)
|
|
|
β-keto acetals. I. Synthesis of pyrazoles and pyrimidines and the steric inhibition of resonance in 5-alkyl-1-p-nitrophenylpyrazoles. Burness DM.
J. Org. Chem. 21(1) , 97-101., (1956)
|
| 1,1-Dimethoxybutan-3-one |
| MFCD00008789 |
| 4,4-Dimethoxybutan-2-one |
| 3-Ketobutyraldehyde dimethyl acetal |
| EINECS 226-605-1 |
| 2-Butanone, 4,4-dimethoxy- |
| β-Oxobutyraldehyde dimethyl acetal |
| 1,1-Dimethoxy-3-butanone |
| 4,4-Dimethoxy-2-butanone |
| Acetylacetaldehyde dimethylacetal |
| Acetylacetaldehydedimethylacetal |
 CAS#:1833-53-0
CAS#:1833-53-0 CAS#:149-73-5
CAS#:149-73-5 CAS#:67-56-1
CAS#:67-56-1 CAS#:7119-27-9
CAS#:7119-27-9 CAS#:78-94-4
CAS#:78-94-4 CAS#:51731-17-0
CAS#:51731-17-0 CAS#:67-64-1
CAS#:67-64-1 CAS#:107-31-3
CAS#:107-31-3 CAS#:56699-02-6
CAS#:56699-02-6 CAS#:56-23-5
CAS#:56-23-5![7-methyl-4H-thieno[2,3-b]pyridine-5-carboxamide structure](https://image.chemsrc.com/caspic/283/108460-23-7.png) CAS#:108460-23-7
CAS#:108460-23-7![6-carbamoyl 4,7-dihydro thieno[3,2-b]pyridine structure](https://image.chemsrc.com/caspic/245/108460-24-8.png) CAS#:108460-24-8
CAS#:108460-24-8 CAS#:109962-22-3
CAS#:109962-22-3 CAS#:150256-11-4
CAS#:150256-11-4 CAS#:31525-67-4
CAS#:31525-67-4 CAS#:4241-27-4
CAS#:4241-27-4 CAS#:4652-27-1
CAS#:4652-27-1 CAS#:3284-51-3
CAS#:3284-51-3 CAS#:33021-53-3
CAS#:33021-53-3 CAS#:5765-44-6
CAS#:5765-44-6
