2-Methyl-1H-indole-3-carbaldehyde

2-Methyl-1H-indole-3-carbaldehyde structure
|
Common Name | 2-Methyl-1H-indole-3-carbaldehyde | ||
|---|---|---|---|---|
| CAS Number | 5416-80-8 | Molecular Weight | 159.185 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 344.3±22.0 °C at 760 mmHg | |
| Molecular Formula | C10H9NO | Melting Point | 200-201 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 169.9±29.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 2-Methylindole-3-carboxaldehyde |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 344.3±22.0 °C at 760 mmHg |
| Melting Point | 200-201 °C(lit.) |
| Molecular Formula | C10H9NO |
| Molecular Weight | 159.185 |
| Flash Point | 169.9±29.8 °C |
| Exact Mass | 159.068420 |
| PSA | 32.86000 |
| LogP | 2.14 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.699 |
| InChIKey | CYZIVXOEJNAIBS-UHFFFAOYSA-N |
| SMILES | Cc1[nH]c2ccccc2c1C=O |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Synthesis and antifungal activity of novel streptochlorin analogues.
Eur. J. Med. Chem. 92 , 776-83, (2015) Streptochlorin, first isolated as a new antibiotic in 1988 from the lipophilic extracts of the mycelium of a Streptomyces sp, is an indole natural products with a variety of biological activities. Bas... |
|
|
Functionalization of benzylic C(sp(3))-H bonds of heteroaryl aldehydes through N-heterocyclic carbene organocatalysis.
Angew. Chem. Int. Ed. Engl. 52(42) , 11134-7, (2013) Aryl aldehyde activation: Oxidative activation of 2-methylindole-3-carboxaldehyde (I) through N-heterocyclic carbene (NHC) organocatalysis generates heterocyclic ortho-quinodimethane (II) as a key int... |
|
|
(2-Methyl-1-phenyl-sulfonyl-1H-indol-3-yl)methanol.
Acta Crystallogr. Sect. E Struct. Rep. Online 64(2) , o542-o542, (2008) In the title compound, C(16)H(15)NO(3)S, the plane of the phenyl ring forms a dihedral angle of 80.37 (8)° with the indole ring system. The crystal packing is stabilized by weak O-H⋯O hydrogen bonds w... |
| Indole-3-carboxaldehyde, 2-methyl- |
| 3-FORMYL-2-METHYLINDOLE |
| 2-Methylindole-3-carboxaldehyde |
| 2-Methyl-1H-indole-3-carbaldehyde |
| MFCD00012077 |
| EINECS 226-512-6 |
| 1H-Indole-3-carboxaldehyde, 2-methyl- |
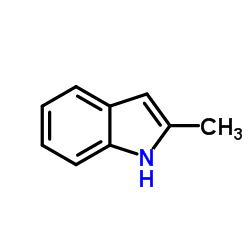 CAS#:95-20-5
CAS#:95-20-5 CAS#:68-12-2
CAS#:68-12-2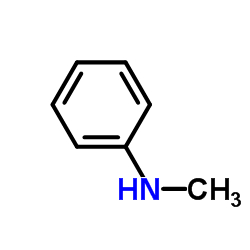 CAS#:100-61-8
CAS#:100-61-8 CAS#:119910-45-1
CAS#:119910-45-1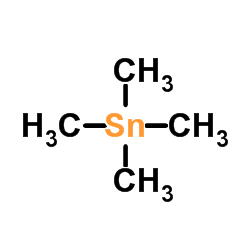 CAS#:594-27-4
CAS#:594-27-4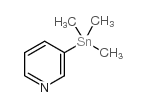 CAS#:59020-09-6
CAS#:59020-09-6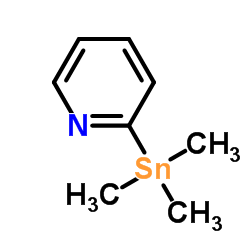 CAS#:13737-05-8
CAS#:13737-05-8 CAS#:67-66-3
CAS#:67-66-3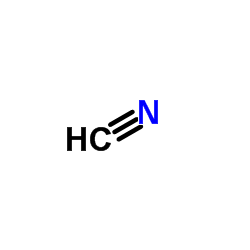 CAS#:74-90-8
CAS#:74-90-8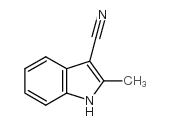 CAS#:51072-83-4
CAS#:51072-83-4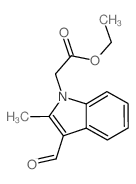 CAS#:433307-59-6
CAS#:433307-59-6 CAS#:38292-40-9
CAS#:38292-40-9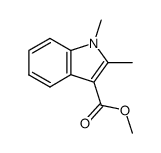 CAS#:54109-65-8
CAS#:54109-65-8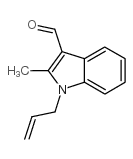 CAS#:230283-19-9
CAS#:230283-19-9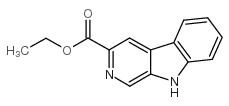 CAS#:74214-62-3
CAS#:74214-62-3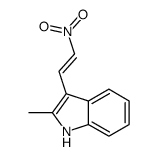 CAS#:2826-91-7
CAS#:2826-91-7 CAS#:91-55-4
CAS#:91-55-4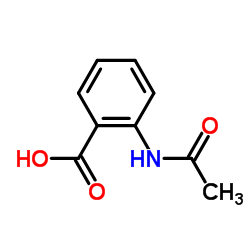 CAS#:89-52-1
CAS#:89-52-1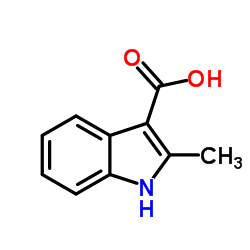 CAS#:63176-44-3
CAS#:63176-44-3
