Glycyrrhizic acid ammonium salt
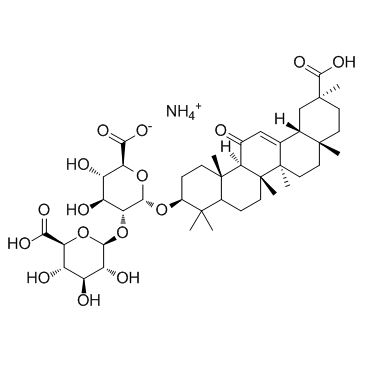
Glycyrrhizic acid ammonium salt structure
|
Common Name | Glycyrrhizic acid ammonium salt | ||
|---|---|---|---|---|
| CAS Number | 53956-04-0 | Molecular Weight | 839.96 | |
| Density | 1.43g/cm3 | Boiling Point | 971.4ºC at 760mmHg | |
| Molecular Formula | C42H65NO16 | Melting Point | 209ºC | |
| MSDS | Chinese USA | Flash Point | 288.1ºC | |
Use of Glycyrrhizic acid ammonium saltMonoammonium glycyrrhizinate hydrate has various pharmacological actions such as anti-inflammatory, antiallergic, antigastriculcer, and antihepatitis activities. |
| Name | Glycyrrhizic acid ammonium salt |
|---|---|
| Synonym | More Synonyms |
| Description | Monoammonium glycyrrhizinate hydrate has various pharmacological actions such as anti-inflammatory, antiallergic, antigastriculcer, and antihepatitis activities. |
|---|---|
| Related Catalog | |
| In Vivo | The increase of the lung W/D weight ratios is significantly reduced by high and medium dose of MAG (10 and 30mg/kg) administration. Pretreatment with MAG (10 and 30mg/kg) efficiently reduces the production of TNF-α and IL-1β. MAG (10, 30mg/kg) significantly decreases NF-κB p65 protein expression, compared with LPS. On the contrary, LPS significantly reduces IκB-α protein expression compared with the control group, whereas MAG (10 and 30mg/kg) significantly increased IκB-α expression, compared with the LPS group[1]. Low- and high-dose MAG treatment significantly reduces the AST, ALT, TBIL, and TBA levels at 14 and 21 d time points when compared with that of the RIF and INH group, suggesting the protective effect of MAG on RIF- and INH-induced liver injury. MAG treatment groups elevate the hepatic GSH level at 7, 14, and 21 d time points and markedly reduce the MDA level at 14 and 21 d time points in RIF- and INH-treated rats, suggesting the protective effect of MAG in RIF- and INH induced liver injuries[2]. |
| Animal Admin | Mice[1] In this study, BALB/c mice (male, 6-8weeks old, and 20-25 g) are used. Mice are randomly divided into five groups: control group, LPS group, and LPS + Monoammonium glycyrrhizinate (MAG: 3, 10, and 30mg/kg) groups. Each group contains eight mice. Mice are anesthetized with intraperitoneal injection of sodium pentobarbital (50mg/kg). Before inducing acute lung injury, the mice are given intraperitoneal injection with MAG (3, 10, and 30mg/kg). One hour later, LPS (5mg/kg) is instilled intratracheally to induce acute lung injury. Normal mice are given PBS[1]. Rats[2] Male Wistar rats (180-220 g) are used. Rats are randomly divided into four groups, i.e., control group, RIF and INH group, MAG low-dose group, and MAG high-dose group, each group has 15 rats. Rats in the RIF and INH group receive RIF (60 mg/kg) and INH (60 mg/kg) by gavage administration once daily; rats in MAG groups are pretreated with MAG at the doses of 45 or 90 mg/kg, RIF (60 mg/kg) and INH (60 mg/kg) are given 3 h after MAG administration; rats in the control group are treated with saline. To evaluate the dynamic effect of drugs, rats in each group are sacrificed on 7, 14, and 21 d after drug administration[2]. |
| References |
| Density | 1.43g/cm3 |
|---|---|
| Boiling Point | 971.4ºC at 760mmHg |
| Melting Point | 209ºC |
| Molecular Formula | C42H65NO16 |
| Molecular Weight | 839.96 |
| Flash Point | 288.1ºC |
| PSA | 272.70000 |
| LogP | 0.32860 |
| Index of Refraction | 49 ° (C=1.5, EtOH) |
| InChIKey | NNMYEXLTVQXZCA-VLQRKCJKSA-N |
| SMILES | CC1(C(=O)O)CCC2(C)CCC3(C)C(=CC(=O)C4C5(C)CCC(OC6OC(C(=O)O)C(O)C(O)C6OC6OC(C(=O)O)C(O)C(O)C6O)C(C)(C)C5CCC43C)C2C1.N.O |
| Storage condition | 2-8°C |
| Water Solubility | Slightly soluble in water, very slightly soluble in anhydrous ethanol, practically insoluble in acetone. It dissolves in dilute solutions of acids and of alkali hydroxides. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| RIDADR | UN 3077 9 / PGIII |
| WGK Germany | 2 |
| RTECS | LZ6500000 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
|
The HMGB1 protein sensitizes colon carcinoma cells to cell death triggered by pro-apoptotic agents.
Int. J. Oncol. 46(2) , 667-76, (2014) The HMGB1 protein has multiple functions in tumor biology and can act both as a transcription factor and as a cytokine. HMGB1 is released during cell death, and in our previous studies we demonstrated... |
|
|
TLR9 activation is triggered by the excess of stimulatory versus inhibitory motifs present in Trypanosomatidae DNA.
PLoS Negl. Trop. Dis. 8(11) , e3308, (2014) DNA sequences purified from distinct organisms, e.g. non vertebrate versus vertebrate ones, were shown to differ in their TLR9 signalling properties especially when either mouse bone marrow-derived- o... |
|
|
Glycyrrhizin reduces HMGB1 secretion in lipopolysaccharide-activated RAW 264.7 cells and endotoxemic mice by p38/Nrf2-dependent induction of HO-1.
Int. Immunopharmacol. 26 , 112-8, (2015) High mobility group box 1 (HMGB1) is now recognized as a late mediator of sepsis. Although glycyrrhizin was known as inhibitor of HMGB1, it is not yet clear underlying mechanism(s). We found that glyc... |
| GLYCAMIL |
| Ammoniumglycynhizinato |
| glycyrrhizic acid monoammonium salt |
| ammonium glycyrrhizinate |
| MFCD00167400 |
| Glycyrrhizin Monoammonium Salt Hydrate |
| Glycyrrhizic Acid Monoammonium Salt Hydrate |
| (3β)-30-Hydroxy-11,30-dioxoolean-12-en-3-yl 2-O-β-D-glucopyranuronosyl-α-D-glucopyranosiduronic acid diammoniate |
| GLYCYRRHIZICAMMONIUM |
| Magnasweet |
| ammoniate |
| Monoammonium Glycyrrhizinate Hydrate |
| Glycyrrhizate monoammonium |
| Glycyrrhizin ammonium |
| Olean-12-en-30-oic acid, 3-[(2-O-β-D-glucopyranuronosyl-α-D-glucopyranuronosyl)oxy]-11-oxo-, diammonium salt, (3β)- |
| Glycyrrhiz |
| AMMONIUMGLYCYRRHIZIN |
| Ammonium glycyrrhizate |
| GLYCYRRHIZIC ACID,NH4 |
| ammoniumglycyrrhizate |
| Monoammoniumglycyrrhizinate |
| Glycyrrhizic acid monoammonium salt trihydrate |
| Olean-12-en-30-oic acid, 3-[(2-O-β-D-glucopyranuronosyl-α-D-glucopyranuronosyl)oxy]-11-oxo-, ammonium salt, (3β)- |
| glycyrram |
| EINECS 258-887-7 |
 CAS#:471-53-4
CAS#:471-53-4
