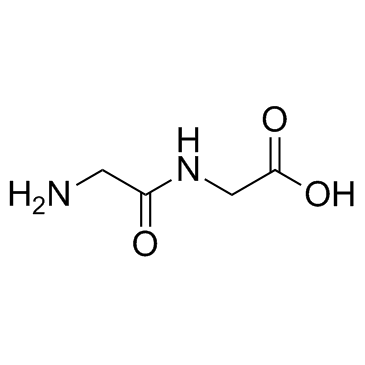
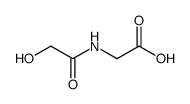
N-Oxalylglycine

N-Oxalylglycine structure
|
Common Name | N-Oxalylglycine | ||
|---|---|---|---|---|
| CAS Number | 5262-39-5 | Molecular Weight | 147.09 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C4H5NO5 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of N-OxalylglycineN-Oxalylglycine (N-Oxaloglycine) is a broad-spectrum 2-oxoglutarate oxygenase inhibitor. N-Oxalylglycine can be used in studies on the hypoxic response and chromatin modifications in animals[1][1]. |
| Name | N-oxalylglycine |
|---|---|
| Synonym | More Synonyms |
| Description | N-Oxalylglycine (N-Oxaloglycine) is a broad-spectrum 2-oxoglutarate oxygenase inhibitor. N-Oxalylglycine can be used in studies on the hypoxic response and chromatin modifications in animals[1][1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Molecular Formula | C4H5NO5 |
| Molecular Weight | 147.09 |
| Exact Mass | 147.016769 |
| PSA | 103.70000 |
| LogP | -1.61 |
| Index of Refraction | 1.521 |
| Storage condition | 2-8°C |
| Water Solubility | deionized water: >10mg/mL |
|
~55% 
N-Oxalylglycine CAS#:5262-39-5 |
| Literature: Cunliffe; Franklin; Hales; Hill Journal of Medicinal Chemistry, 1992 , vol. 35, # 14 p. 2652 - 2658 |
|
~% 
N-Oxalylglycine CAS#:5262-39-5 |
| Literature: Mecinovic, Jasmin; Hamed, Refaat B.; Schofield, Christopher J. Angewandte Chemie, International Edition, 2009 , vol. 48, # 15 p. 2796 - 2800 |
|
~% 
N-Oxalylglycine CAS#:5262-39-5 |
| Literature: Kraemer Chemische Berichte, 1906 , vol. 39, p. 4386 Full Text Show Details Pollack B.Ph.P., 1906 , vol. 7, p. 17 |
|
~% 
N-Oxalylglycine CAS#:5262-39-5 |
| Literature: Oparin Biochemische Zeitschrift, 1921 , vol. 124, p. 92 Izv.ross.Akad., vol. <6>16, p. 543 Chem. Zentralbl., 1925 , vol. 96, # II p. 728 |
|
~% 
N-Oxalylglycine CAS#:5262-39-5 |
| Literature: Viscontini Helvetica Chimica Acta, 1946 , vol. 29, p. 1496 |
|
~% 
N-Oxalylglycine CAS#:5262-39-5 |
| Literature: Kerp; Unger Chemische Berichte, 1897 , vol. 30, p. 590 Full Text Show Details Kraemer Chemische Berichte, 1906 , vol. 39, p. 4386 |
|
~% 
N-Oxalylglycine CAS#:5262-39-5 |
| Literature: Viscontini Helvetica Chimica Acta, 1946 , vol. 29, p. 1496 |
|
~% 
N-Oxalylglycine CAS#:5262-39-5 |
| Literature: Kerp; Unger Chemische Berichte, 1897 , vol. 30, p. 590 |
|
~% 
N-Oxalylglycine CAS#:5262-39-5 |
| Literature: Oparin Biochemische Zeitschrift, 1921 , vol. 124, p. 92 Izv.ross.Akad., vol. <6>16, p. 543 Chem. Zentralbl., 1925 , vol. 96, # II p. 728 |
| HS Code | 2918300090 |
|---|---|
| Summary | 2918300090 other carboxylic acids with aldehyde or ketone function but without other oxygen function, their anhydrides, halides, peroxides, peroxyacids and their derivatives。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
| N-Oxalylglycine |
| N-OXALYOLGLYCINE |
| Glycine, N-(carboxycarbonyl)- |
| N-(Carboxycarbonyl)glycine |
| OGA |
| Oxaloglycine |
| N-oxalyl glycine,1a |
| 2-oxo-3-azaglutaric acid |
| 2-(carboxymethylamino)-2-oxoacetic acid |
| N-Hydroxyoxalyl-glycin |
| N-hydroxyoxalyl-glycine |
| Oxalylglycine |