7-Bromo-1H-indole
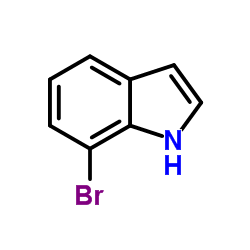
7-Bromo-1H-indole structure
|
Common Name | 7-Bromo-1H-indole | ||
|---|---|---|---|---|
| CAS Number | 51417-51-7 | Molecular Weight | 196.044 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 316.9±15.0 °C at 760 mmHg | |
| Molecular Formula | C8H6BrN | Melting Point | 41-44 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 145.5±20.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 7-Bromo-1H-indole7-Bromo-1H-indole is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 7-Bromoindole |
|---|---|
| Synonym | More Synonyms |
| Description | 7-Bromo-1H-indole is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 316.9±15.0 °C at 760 mmHg |
| Melting Point | 41-44 °C(lit.) |
| Molecular Formula | C8H6BrN |
| Molecular Weight | 196.044 |
| Flash Point | 145.5±20.4 °C |
| Exact Mass | 194.968353 |
| PSA | 15.79000 |
| LogP | 2.91 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.712 |
| InChIKey | RDSVSEFWZUWZHW-UHFFFAOYSA-N |
| SMILES | Brc1cccc2cc[nH]c12 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R20/21/22;R36/37/38 |
| Safety Phrases | S26-S36-S36/37/39-S22 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 29339990 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Multicomponent phenol hydroxylase-catalysed formation of hydroxyindoles and dyestuffs from indole and its derivatives.
Lett. Appl. Microbiol. 41(2) , 163-8, (2005) To establish multicomponent phenol hydroxylases (mPHs) as novel biocatalysts for producing dyestuffs and hydroxyindoles such as 7-hydroxyindole (7-HI) from indole and its derivatives.We have isolated ... |
|
|
Indole and 7-benzyloxyindole attenuate the virulence of Staphylococcus aureus.
Appl. Microbiol. Biotechnol. 97(10) , 4543-52, (2013) Human pathogens can readily develop drug resistance due to the long-term use of antibiotics that mostly inhibit bacterial growth. Unlike antibiotics, antivirulence compounds diminish bacterial virulen... |
|
|
Diversity Oriented Synthesis: Concise Entry to Novel Derivatives of Yohimbine and Corynanthe Alkaloids.
Tetrahedron Lett. 53(5) , 477-479, (2012) A novel MCAP-cycloaddition sequence has been applied to the facile synthesis of _-carboline intermediates to gain rapid access to novel derivatives of yohimbine-like and corynanthe-like compounds that... |
| 7-Bromoindole |
| 1H-Indole,7-broMo |
| 3-CYANO-7-CHLORO INDOLE |
| 7-bromoidnole |
| 7-Brom-indol |
| 7-BROMOCHROMANONE |
| 7-bromo indole |
| MFCD00799492 |
| 1H-Indole, 7-bromo- |
| 7-Bromo-1H-indole |
| 7-broMo-4H-indole |
| 7-BroMoindole 250MG |
| RARECHEM AH BS 0083 |
 CAS#:577-19-5
CAS#:577-19-5 CAS#:1826-67-1
CAS#:1826-67-1 CAS#:62813-85-8
CAS#:62813-85-8 CAS#:74798-64-4
CAS#:74798-64-4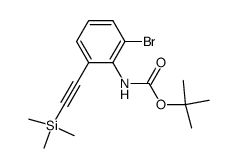 CAS#:187099-50-9
CAS#:187099-50-9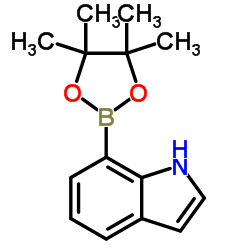 CAS#:642494-37-9
CAS#:642494-37-9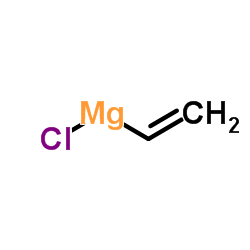 CAS#:3536-96-7
CAS#:3536-96-7 CAS#:615-36-1
CAS#:615-36-1 CAS#:104682-95-3
CAS#:104682-95-3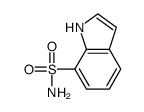 CAS#:111048-64-7
CAS#:111048-64-7 CAS#:189266-03-3
CAS#:189266-03-3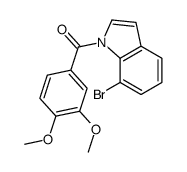 CAS#:194231-75-9
CAS#:194231-75-9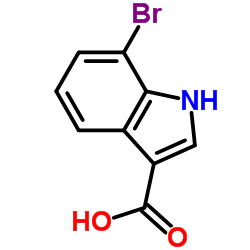 CAS#:86153-25-5
CAS#:86153-25-5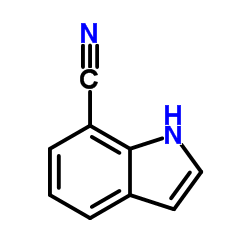 CAS#:96631-87-7
CAS#:96631-87-7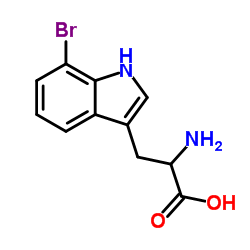 CAS#:852391-45-8
CAS#:852391-45-8 CAS#:93247-78-0
CAS#:93247-78-0 CAS#:210889-31-9
CAS#:210889-31-9 CAS#:280752-68-3
CAS#:280752-68-3
