Verminoside
Modify Date: 2025-08-25 08:36:04
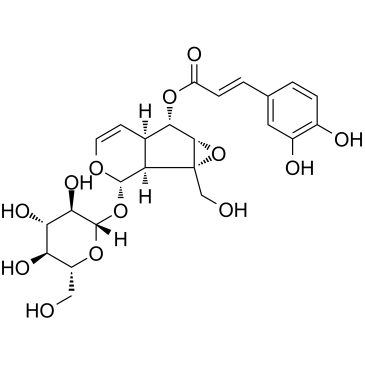
Verminoside structure
|
Common Name | Verminoside | ||
|---|---|---|---|---|
| CAS Number | 50932-19-9 | Molecular Weight | 492.473 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 739.1±60.0 °C at 760 mmHg | |
| Molecular Formula | C24H28O13 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 253.2±26.4 °C | |
Use of VerminosideVerminoside is an iridoid isolated from Kigelia africana, exhibits anti-inflammatory and remarkable antioxidant activity with a radical-scavenging activity of 2.5 μg/mL. The genotoxicity of Verminoside on human lymphocytes is associated with elevated levels of PARP-1 and p53 proteins[1][2][3]. |
| Name | (1aS,1bS,2S,5aR,6S,6aS)-2-(β-D-Glucopyranosyloxy)-1a-(hydroxymeth yl)-1a,1b,2,5a,6,6a-hexahydrooxireno[4,5]cyclopenta[1,2-c]pyran-6 -yl (2E)-3-(3,4-dihydroxyphenyl)acrylate |
|---|---|
| Synonym | More Synonyms |
| Description | Verminoside is an iridoid isolated from Kigelia africana, exhibits anti-inflammatory and remarkable antioxidant activity with a radical-scavenging activity of 2.5 μg/mL. The genotoxicity of Verminoside on human lymphocytes is associated with elevated levels of PARP-1 and p53 proteins[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
PARP-1 |
| In Vitro | Verminoside (Compound 1; 0.01-1 mM; 25 hours; J774.A1 macrophages) treatment shows significant and concentration-related inhibition of iNOS expression at 0.1 mM and 1 mM in LPS-stimulated J774.A1 macrophages[1]. Western Blot Analysis[1] Cell Line: J774.A1 macrophages Concentration: 0.01 mM, 0.1 mM or 1 mM Incubation Time: 25 hours Result: Showed significant and concentration-related inhibition of iNOS expression at 0.1 mM and 1 mM in LPS-stimulated J774.A1 macrophages. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 739.1±60.0 °C at 760 mmHg |
| Molecular Formula | C24H28O13 |
| Molecular Weight | 492.473 |
| Flash Point | 253.2±26.4 °C |
| Exact Mass | 492.163147 |
| PSA | 208.13000 |
| LogP | -1.81 |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.671 |
| picroside-1 |
| PicrosideI |
| 2-Propenoic acid, 3-phenyl-, (1aS,1bS,2S,5aR,6S,6aS)-2-(β-D-glucopyranosyloxy)-1a,1b,2,5a,6,6a-hexahydro-1a-(hydroxymethyl)oxireno[4,5]cyclopenta[1,2-c]pyran-6-yl ester, (2E)- |
| β-D-Glucopyranoside, (1aS,1bS,2S,5aR,6S,6aS)-1a,1b,2,5a,6,6a-hexahydro-6-hydroxy-1a-(hydroxymethyl)oxireno[4,5]cyclopenta[1,2-c]pyran-2-yl 6-O-[(2E)-1-oxo-3-phenyl-2-propen-1-yl]- |
| 2-Propenoic acid, 3-(3,4-dihydroxyphenyl)-, (1aS,1bS,2S,5aR,6S,6aS)-2-(β-D-glucopyranosyloxy)-1a,1b,2,5a,6,6a-hexahydro-1a-(hydroxymethyl)oxireno[4,5]cyclopenta[1,2-c]pyran-6-yl ester, (2E)- |
| 6'-O-trans-cinnamoylcatalpol |
| (1aS,1bS,2S,5aR,6S,6aS)-6-Hydroxy-1a-(hydroxymethyl)-1a,1b,2,5a,6,6a-hexahydrooxireno[4,5]cyclopenta[1,2-c]pyran-2-yl 6-O-[(2E)-3-phenyl-2-propenoyl]-β-D-glucopyranoside |
| picrosides I |
| Picroside I |
| (1aS,1bS,2S,5aR,6S,6aS)-2-(β-D-Glucopyranosyloxy)-1a-(hydroxymethyl)-1a,1b,2,5a,6,6a-hexahydrooxireno[4,5]cyclopenta[1,2-c]pyran-6-yl (2E)-3-(3,4-dihydroxyphenyl)acrylate |
| (1aS,1bS,2S,5aR,6S,6aS)-2-(β-D-Glucopyranosyloxy)-1a-(hydroxymethyl)-1a,1b,2,5a,6,6a-hexahydrooxireno[4,5]cyclopenta[1,2-c]pyran-6-yl (2E)-3-phenylacrylate |
| Verminoside |

