Mizoribine
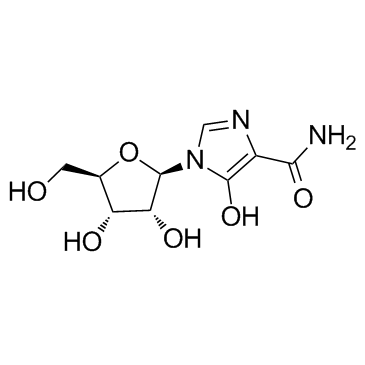
Mizoribine structure
|
Common Name | Mizoribine | ||
|---|---|---|---|---|
| CAS Number | 50924-49-7 | Molecular Weight | 259.216 | |
| Density | 2.1±0.1 g/cm3 | Boiling Point | 755.9±60.0 °C at 760 mmHg | |
| Molecular Formula | C9H13N3O6 | Melting Point | >200ºC | |
| MSDS | Chinese USA | Flash Point | 410.9±32.9 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of MizoribineMizoribine(NSC 289637; HE 69; β-Bredinin) is an immunosuppressive agents (IC50=100 uM) that inhibit the proliferation of lymphocytes selectively, via inhibition of IMPDH.IC50 Value:Target: IMPDHin vitro: Unlike azathioprine, Mizoribine is not taken up by nucleic acids in the cell. Instead, after phosphorylation MZR-5 -monophosphate inhibits GMP synthesis by the antagonistic blocking of IMPDH (Ki = 10(-8)M) and GMP- synthetase (Ki =10(-5) M) [1]. Pretreatment of cells with MZR partially, but significantly, attenuates the expression of monocyte chemoattractant protein (MCP)-1 mRNA and protein, whereas the poly IC-induced expressions for the other functional molecules, such as CCL5, fractalkine and IL-8 were not influenced by MZR treatment [2].in vivo:MZR 150 mg was administered once a day. After 6 months, the remission rate was 72.7% (2 subjects achieved complete remission, and 9 partial remission). After 3 and 6 months, significant reductions (p < 0.01) were obtained in 24-h proteinuria (g/day) [3]. |
| Name | 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-hydroxyimidazole-4-carboxamide |
|---|---|
| Synonym | More Synonyms |
| Description | Mizoribine(NSC 289637; HE 69; β-Bredinin) is an immunosuppressive agents (IC50=100 uM) that inhibit the proliferation of lymphocytes selectively, via inhibition of IMPDH.IC50 Value:Target: IMPDHin vitro: Unlike azathioprine, Mizoribine is not taken up by nucleic acids in the cell. Instead, after phosphorylation MZR-5 -monophosphate inhibits GMP synthesis by the antagonistic blocking of IMPDH (Ki = 10(-8)M) and GMP- synthetase (Ki =10(-5) M) [1]. Pretreatment of cells with MZR partially, but significantly, attenuates the expression of monocyte chemoattractant protein (MCP)-1 mRNA and protein, whereas the poly IC-induced expressions for the other functional molecules, such as CCL5, fractalkine and IL-8 were not influenced by MZR treatment [2].in vivo:MZR 150 mg was administered once a day. After 6 months, the remission rate was 72.7% (2 subjects achieved complete remission, and 9 partial remission). After 3 and 6 months, significant reductions (p < 0.01) were obtained in 24-h proteinuria (g/day) [3]. |
|---|---|
| Related Catalog | |
| References |
[1]. Ishikawa H. Mizoribine and mycophenolate mofetil. Curr Med Chem. 1999 Jul;6(7):575-97. |
| Density | 2.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 755.9±60.0 °C at 760 mmHg |
| Melting Point | >200ºC |
| Molecular Formula | C9H13N3O6 |
| Molecular Weight | 259.216 |
| Flash Point | 410.9±32.9 °C |
| Exact Mass | 259.080444 |
| PSA | 151.06000 |
| LogP | -0.17 |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C |
| Index of Refraction | 1.795 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H315-H319-H335-H360 |
| Precautionary Statements | P201-P261-P305 + P351 + P338-P308 + P313 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R46 |
| Safety Phrases | 53-22-26-36/37/39-45 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | NI3980000 |
|
Successful multitarget therapy using mizoribine and tacrolimus for refractory Takayasu arteritis.
Rheumatology (Oxford.) 53(8) , 1530-2, (2014)
|
|
|
[Treatment of rheumatic diseases: current status and future prospective. Topics: II. Immunosuppressant/antirheumatic drugs; 5. Leflunomide and mizoribine].
Nippon. Naika Gakkai Zasshi. 100(10) , 2929-35, (2011)
|
|
|
Spantide II, a novel tachykinin antagonist having high potency and low histamine-releasing effect.
Clin. Transplant. 10 , 116, (1996) Two undecapeptide substance P (SP) analogues, Spantide I and Spantide II, were tested for their capacity to block the contractile effect of SP on the guinea pig isolated taenia coli and the contractil... |
| 5-Hydroxy-1-(β-D-ribofuranosyl)-1H-imidazole-4-carboxamide |
| HE-69 |
| Mizoribinum |
| Bredinin |
| 1H-Imidazole-4-carboxamide, 5-hydroxy-1-β-D-ribofuranosyl- |
| Mizoribina |
| Mizoribine |
| 5-Hydroxy-1-b-D-ribofuranosyl-1H-imidazole-4-carboxamide |
| Bredinine |
| 4-Carbamoyl-1-(β-D-ribofuranosyl)-1H-imidazol-3-ium-5-olate |
| 4-Carbamoyl-1-b-D-ribofuranosylimidazolium-5-olate |
| Mizoribine [INN:JAN] |
| MFCD00057221 |
| Mizoribina [INN-Spanish] |
| Mizoribinum [INN-Latin] |
| 1-(β-D-Ribofuranosyl)-5-hydroxyimidazole-4-carboxamide |

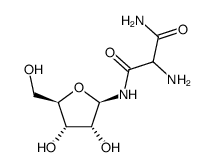

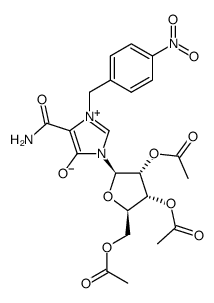
![((3aR,4R,6R,6aR)-6-(4-carbamoyl-5-hydroxy-1H-imidazol-1-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl acetate structure](https://image.chemsrc.com/caspic/077/168906-86-3.png)
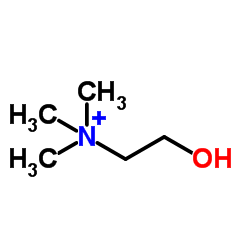 CAS#:62-49-7
CAS#:62-49-7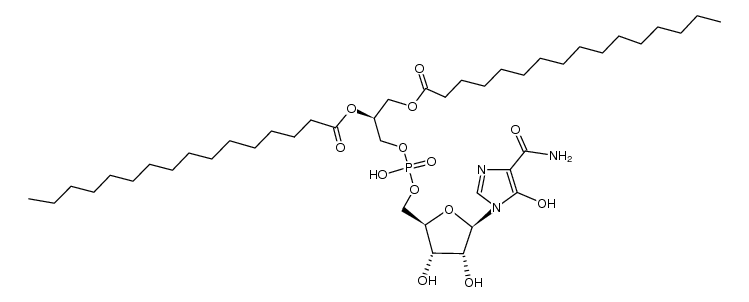 CAS#:107110-35-0
CAS#:107110-35-0