CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
KE1050000
-
CHEMICAL NAME :
-
Ergocalciferol
-
CAS REGISTRY NUMBER :
-
50-14-6
-
LAST UPDATED :
-
199701
-
DATA ITEMS CITED :
-
27
-
MOLECULAR FORMULA :
-
C28-H44-O
-
MOLECULAR WEIGHT :
-
396.72
-
WISWESSER LINE NOTATION :
-
L56 FYTJ A1 BY1&1U1Y1&Y1&1 FU2U- BL6YYTJ AU1 DQ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
12600 ug/kg/72W
-
TOXIC EFFECTS :
-
Behavioral - anorexia (human) Gastrointestinal - nausea or vomiting Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
LANCAO Lancet. (7 Adam St., London WC2N 6AD, UK) V.1- 1823- Volume(issue)/page/year: 1,1164,1980
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
10 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
85ARAE "Agricultural Chemicals," Thomson, W.T., 4 vols., Fresno, CA, Thomson Publications, 1976/77 revision Volume(issue)/page/year: 3,132,1986
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
23700 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
PEMNDP Pesticide Manual. (The British Crop Protection Council, 20 Bridport Rd., Thornton Heath CR4 7QG, UK) V.1- 1968- Volume(issue)/page/year: 9,115,1991
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
4 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Gastrointestinal - hypermotility, diarrhea Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
ZGEMAZ Zeitschrift fuer die Gesamte Experimentelle Medizin. (Berlin, Fed. Rep. Ger.) V.1-139, 1913-65. For publisher information, see REXMAS. Volume(issue)/page/year: 116,138,1950
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
10 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - fluid intake Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
ZGEMAZ Zeitschrift fuer die Gesamte Experimentelle Medizin. (Berlin, Fed. Rep. Ger.) V.1-139, 1913-65. For publisher information, see REXMAS. Volume(issue)/page/year: 116,138,1950
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
5 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
ZGEMAZ Zeitschrift fuer die Gesamte Experimentelle Medizin. (Berlin, Fed. Rep. Ger.) V.1-139, 1913-65. For publisher information, see REXMAS. Volume(issue)/page/year: 116,138,1950
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
5 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
ZGEMAZ Zeitschrift fuer die Gesamte Experimentelle Medizin. (Berlin, Fed. Rep. Ger.) V.1-139, 1913-65. For publisher information, see REXMAS. Volume(issue)/page/year: 116,138,1950
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
5 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,128,1982
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
40 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,128,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Bird - duck
-
DOSE/DURATION :
-
>2 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
DEVEAA Defense des Vegetaux. (Federation Nationale des Groupements de Protection des Cultures, 149, rue de Bercy, 75595 Paris Cedex, 12, France) V.1- 1947- Volume(issue)/page/year: 43(255-256),14,1989 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
660 mg/kg/7D-I
-
TOXIC EFFECTS :
-
Cardiac - other changes Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis) Kidney, Ureter, Bladder - changes in bladder weight
-
REFERENCE :
-
TOLED5 Toxicology Letters. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1977- Volume(issue)/page/year: 69,257,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
240 ku/kg/12D-I
-
TOXIC EFFECTS :
-
Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Nutritional and Gross Metabolic - changes in calcium
-
REFERENCE :
-
IJMDAI Israel Journal of Medical Sciences. (POB 1435, Jerusalem 91013, Israel) V.1- 1965- Volume(issue)/page/year: 4,827,1968 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
45 mg/kg
-
SEX/DURATION :
-
female 13-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - endocrine system
-
REFERENCE :
-
ARPAAQ Archives of Pathology. (Chicago, IL) V.5(3)-50(3), 1928-50; V.70-99, 1960-75. For publisher information, see APLMAS. Volume(issue)/page/year: 73,371,1962
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
30 mg/kg
-
SEX/DURATION :
-
female 10-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4)
-
REFERENCE :
-
45REAG "Teratology of the Limbs, Symposium on Prenatal Development, 4th, 1980," Merker, H.J., et al., eds., Berlin, Walter de Gruyter and Co., 1980 Volume(issue)/page/year: 4,339,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
55 mg/kg
-
SEX/DURATION :
-
female 9-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
ARPAAQ Archives of Pathology. (Chicago, IL) V.5(3)-50(3), 1928-50; V.70-99, 1960-75. For publisher information, see APLMAS. Volume(issue)/page/year: 87,563,1969
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
33750 ug/kg
-
SEX/DURATION :
-
female 9 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated)
-
REFERENCE :
-
IJMDAI Israel Journal of Medical Sciences. (POB 1435, Jerusalem 91013, Israel) V.1- 1965- Volume(issue)/page/year: 2,14,1966
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
22500 ug/kg
-
SEX/DURATION :
-
female 13-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
ARPAAQ Archives of Pathology. (Chicago, IL) V.5(3)-50(3), 1928-50; V.70-99, 1960-75. For publisher information, see APLMAS. Volume(issue)/page/year: 73,371,1962
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
17500 ug/kg
-
SEX/DURATION :
-
female 1-7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated)
-
REFERENCE :
-
FESTAS Fertility and Sterility. (American Fertility Soc., 608 13th Ave. S, Birmingham, AL 35282) V.1- 1950- Volume(issue)/page/year: 12,343,1961
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
35 mg/kg
-
SEX/DURATION :
-
female 8-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
AEMBAP Advances in Experimental Medicine and Biology. (Plenum Pub. Corp., 233 Spring St., New York, NY 10013) V.1- 1967- Volume(issue)/page/year: 27,219,1972
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
200 mg/kg
-
SEX/DURATION :
-
female 4-7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 26,1589,1976
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
1750 ug/kg
-
SEX/DURATION :
-
female 1-28 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
REFERENCE :
-
PEREBL Pediatric Research. (Williams & Wilkins Co., 428 E. Preston St., Baltimore, MD 21202) V.1- 1967- Volume(issue)/page/year: 13,121,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
9375 ug/kg
-
SEX/DURATION :
-
female 2-31 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain) Reproductive - Effects on Newborn - delayed effects
-
REFERENCE :
-
PEDIAU Pediatrics. (American Academy of Pediatrics, P.O. Box 1034, Evanston, IL 60204) V.1- 1948- Volume(issue)/page/year: 43,12,1969
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
17500 ug/kg
-
SEX/DURATION :
-
female 1-28 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
REFERENCE :
-
PEREBL Pediatric Research. (Williams & Wilkins Co., 428 E. Preston St., Baltimore, MD 21202) V.1- 1967- Volume(issue)/page/year: 13,121,1979 *** REVIEWS *** TOXICOLOGY REVIEW PEDIAU Pediatrics. (American Academy of Pediatrics, P.O. Box 1034, Evanston, IL 60204) V.1- 1948- Volume(issue)/page/year: 43,1,1969 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 80561 No. of Facilities: 245 (estimated) No. of Industries: 4 No. of Occupations: 15 No. of Employees: 8867 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 80561 No. of Facilities: 846 (estimated) No. of Industries: 4 No. of Occupations: 21 No. of Employees: 18224 (estimated) No. of Female Employees: 12361 (estimated)
|
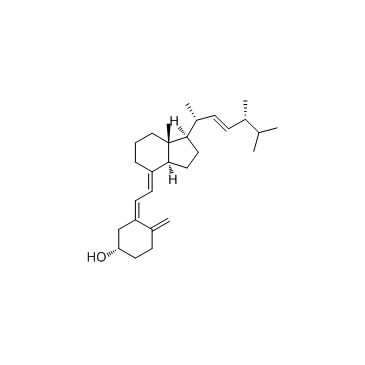






 CAS#:84985-78-4
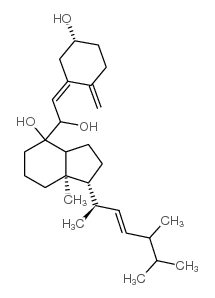
CAS#:84985-78-4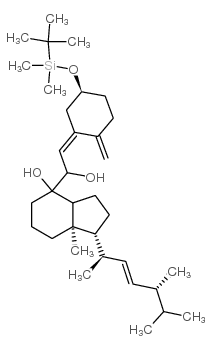 CAS#:131852-63-6
CAS#:131852-63-6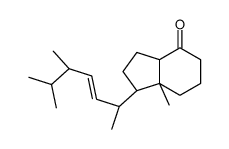 CAS#:55812-80-1
CAS#:55812-80-1![[1R-[1α,(1R*,2E,4R*),3aβ,4β,7aα]]-Octahydro-7a-methyl-1-(1,4,5-trimethylhex-2-enyl)-1H-inden-4-ol Structure](https://image.chemsrc.com/caspic/165/245090-36-2.png) CAS#:245090-36-2
CAS#:245090-36-2![[1R-[1α,(1R*,2E,4R*),3aβ,4α,7aα]]-4-[(Benzothiazol-2-yl)sulfanyl]octahydro-7a-methyl-1-(1,4,5-trimethylhex-2-enyl)-1H-indene Structure](https://image.chemsrc.com/caspic/127/245090-37-3.png) CAS#:245090-37-3
CAS#:245090-37-3 CAS#:57-87-4
CAS#:57-87-4 CAS#:21307-05-1
CAS#:21307-05-1![2-[5-[tert-butyl(dimethyl)silyl]oxy-2-methylidenecyclohexylidene]ethanol Structure](https://image.chemsrc.com/caspic/109/96685-53-9.png) CAS#:96685-53-9
CAS#:96685-53-9![[1R-[1α,(1R*,2E,4R*),3aβ,4α,7aα]]-4-[(Benzothiazol-2-yl)sulfonyl]octahydro-7a-methyl-1-(1,4,5-trimethylhex-2-enyl)-1H-indene Structure](https://image.chemsrc.com/caspic/089/245090-38-4.png) CAS#:245090-38-4
CAS#:245090-38-4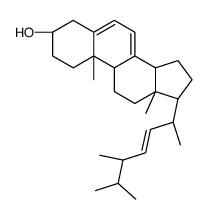 CAS#:474-69-1
CAS#:474-69-1![tert-Butyl-dimethyl-(4-methylene-3-{2-[7a-methyl-1-(1,4,5-trimethyl-hex-2-enyl)-octahydro-inden-4-ylidene]-ethylidene}-cyclohexyloxy)-silane structure](https://image.chemsrc.com/caspic/327/104846-62-0.png) CAS#:104846-62-0
CAS#:104846-62-0![Ethyl (5R)-5-[(1R,4E,7aR)-4-{(2E)-2-[(5S)-5-{[dimethyl(2-methyl-2 -propanyl)silyl]oxy}-2-methylenecyclohexylidene]ethylidene}-7a-me thyloctahydro-1H-inden-1-yl]hexanoate (non-preferred name) structure](https://image.chemsrc.com/caspic/213/147125-14-2.png) CAS#:147125-14-2
CAS#:147125-14-2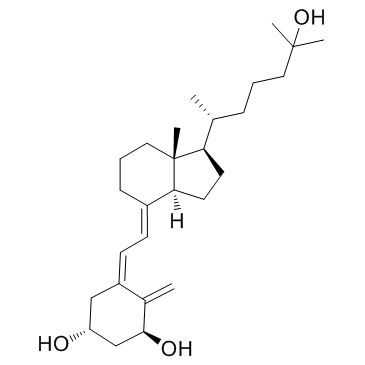 CAS#:32222-06-3
CAS#:32222-06-3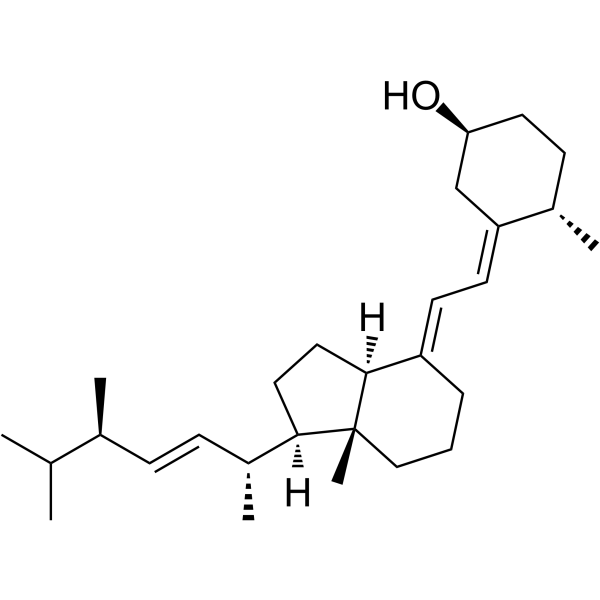 CAS#:67-96-9
CAS#:67-96-9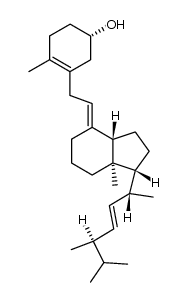 CAS#:104396-98-7
CAS#:104396-98-7 CAS#:54573-75-0
CAS#:54573-75-0 CAS#:64190-52-9
CAS#:64190-52-9 CAS#:66774-80-9
CAS#:66774-80-9![Toluene-4-sulfonic acid2-[4-(tert-butyl-dimethyl-silanyloxy)-7a-methyl-octahydro-inden-1-yl]-propyl ester structure](https://image.chemsrc.com/caspic/047/100928-04-9.png) CAS#:100928-04-9
CAS#:100928-04-9
