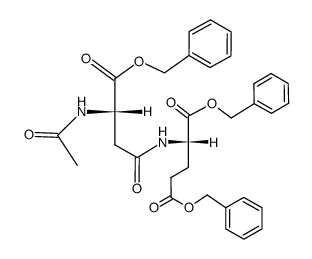
Ac-Asp(Glu-OH)-OH
Ac-Asp(Glu-OH)-OH structure
|
Common Name | Ac-Asp(Glu-OH)-OH | ||
|---|---|---|---|---|
| CAS Number | 4910-46-7 | Molecular Weight | 304.253 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 769.5±60.0 °C at 760 mmHg | |
| Molecular Formula | C11H16N2O8 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 419.1±32.9 °C | |
Use of Ac-Asp(Glu-OH)-OHβ-Spaglumic acid (β-NAAG) is a competitive NAAG peptidase inhibitor (Ki=1 µM) that protects spinal cord neurons from excitotoxicity and hypoxic damage. β-Spaglumic acid is also a selective mGluR3 antagonist (mGluR3 receptor functions to regulate activity-dependent synaptic potentiation in the hippocampus). β-Spaglumic acid can be used in neuroprotection-related studies[1][2]. |
| Name | N-Acetyl-β-Asp-Glu |
|---|---|
| Synonym | More Synonyms |
| Description | β-Spaglumic acid (β-NAAG) is a competitive NAAG peptidase inhibitor (Ki=1 µM) that protects spinal cord neurons from excitotoxicity and hypoxic damage. β-Spaglumic acid is also a selective mGluR3 antagonist (mGluR3 receptor functions to regulate activity-dependent synaptic potentiation in the hippocampus). β-Spaglumic acid can be used in neuroprotection-related studies[1][2]. |
|---|---|
| Related Catalog | |
| Target |
NAAG peptidase, mGluR3[1][2]. |
| In Vitro | β-Spaglumic acid (63-1000 µM; 2 h) protects against NMDA-induced injury of spinal cord cells in a dose-dependent manner[1]. β-Spaglumic acid (0-1000 µM; 2 h) protects spinal cord cells against hypoxia[1]. β-Spaglumic acid (500 µM) significantly reduces intraneuronal free Ca2+ responses upon neuronal exposure to 25 µM NMDA[1]. β-Spaglumic acid (100 µM; 7 min) antagonizes mGluR3 in cerebellar granule cells[2]. Cell Viability Assay[1] Cell Line: Spinal cord cells (NMDA-induced) (from spinal cords removed from prenatal day 15 Sprague-Dawley rat fetuses) Concentration: 63-1000 µM Incubation Time: 2 h Result: Led to a significant attenuation of NMDA toxicity at a concentration of 63 µM and completely blocked NMDA toxicity with 500 and 1000 µM concentrations. Apparently minimized the basal loss of cell viability associated with experimental handling of the cells (e.g. serum removal, media changes). Cell Viability Assay[1] Cell Line: Spinal cord cells (hypoxic-induced) Concentration: 0-1000 µM Incubation Time: 2 h Result: Provided 75% protection during hypoxia when at 8 µM and completely eliminated hypoxia-induced loss of viability (107.4-114.4% protection, respectively) when at 63-1000 µM. Cell Viability Assay[2] Cell Line: Cerebellar granule cells (expressing group I-III mGluRs) Concentration: 100 µM Incubation Time: 7 min Result: Blocked NAAG inhibition of forskolin-stimulated cAMP formation via mGluR3. |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 769.5±60.0 °C at 760 mmHg |
| Molecular Formula | C11H16N2O8 |
| Molecular Weight | 304.253 |
| Flash Point | 419.1±32.9 °C |
| Exact Mass | 304.090668 |
| PSA | 170.10000 |
| LogP | -1.96 |
| Vapour Pressure | 0.0±5.7 mmHg at 25°C |
| Index of Refraction | 1.540 |
| InChIKey | GUCKKCMJTSNWCU-BQBZGAKWSA-N |
| SMILES | CC(=O)NC(CC(=O)NC(CCC(=O)O)C(=O)O)C(=O)O |
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
~87% 
Ac-Asp(Glu-OH)-OH CAS#:4910-46-7 |
| Literature: Piotrovskii, L. B.; Dumpis, M. A.; Poznyakova, L. N.; Aleksandrova, L. N.; Sepetov, N. F. Journal of Organic Chemistry USSR (English Translation), 1988 , vol. 24, p. 96 - 100 Zhurnal Organicheskoi Khimii, 1988 , vol. 24, # 1 p. 111 - 116 |
|
~% 
Ac-Asp(Glu-OH)-OH CAS#:4910-46-7 |
| Literature: Piotrovskii, L. B.; Dumpis, M. A.; Poznyakova, L. N.; Aleksandrova, L. N.; Sepetov, N. F. Journal of Organic Chemistry USSR (English Translation), 1988 , vol. 24, p. 96 - 100 Zhurnal Organicheskoi Khimii, 1988 , vol. 24, # 1 p. 111 - 116 |
|
~% 
Ac-Asp(Glu-OH)-OH CAS#:4910-46-7 |
| Literature: Piotrovskii, L. B.; Dumpis, M. A.; Poznyakova, L. N.; Aleksandrova, L. N.; Sepetov, N. F. Journal of Organic Chemistry USSR (English Translation), 1988 , vol. 24, p. 96 - 100 Zhurnal Organicheskoi Khimii, 1988 , vol. 24, # 1 p. 111 - 116 |
|
~% 
Ac-Asp(Glu-OH)-OH CAS#:4910-46-7 |
| Literature: Piotrovskii, L. B.; Dumpis, M. A.; Poznyakova, L. N.; Aleksandrova, L. N.; Sepetov, N. F. Journal of Organic Chemistry USSR (English Translation), 1988 , vol. 24, p. 96 - 100 Zhurnal Organicheskoi Khimii, 1988 , vol. 24, # 1 p. 111 - 116 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Neural-activity-dependent release of S100B from astrocytes enhances kainate-induced gamma oscillations in vivo.
J. Mol. Endocrinol. 28 , 10928-36, (2008) S100B is the principal calcium-binding protein of astrocytes and known to be secreted to extracellular space. Although secreted S100B has been reported to promote neurite extension and cell survival v... |
|
|
Molecular identification of β-citrylglutamate hydrolase as glutamate carboxypeptidase 3.
J. Biol. Chem. 286 , 38220-38230, (2011) β-Citrylglutamate (BCG), a compound present in adult testis and in the CNS during the pre- and perinatal periods is synthesized by an intracellular enzyme encoded by the RIMKLB gene and hydrolyzed by ... |
|
|
Effect of N-acetylaspartylglutamate (NAAG) on non-quantal and spontaneous quantal release of acetylcholine at the neuromuscular synapse of rat.
J. Neurochem. 94 , 257-267, (2005) N-Acetylaspartylglutamate (NAAG), known to be present in rat motor neurons, may participate in neuronal modulation of non-quantal secretion of acetylcholine (ACh) from motor nerve terminals. Non-quant... |
| N-(N-Acetylaspartyl)glutamic acid |
| N-Acetyl-α-aspartylglutamic acid |
| 2-[(2-Acetamido-3-carboxypropanoyl)amino]pentanedioic acid |
| Glutamic acid, N-acetyl-α-aspartyl- |
| Spaglumic acid,N-Acetyl-L-aspartyl-L-glutamicacid |
| N-Acetyl-a-aspartylglutamic acid |
| Ac-Asp(Glu-OH)-OH |