Bufotenine
Bufotenine structure
|
Common Name | Bufotenine | ||
|---|---|---|---|---|
| CAS Number | 487-93-4 | Molecular Weight | 204.26800 | |
| Density | 1.178g/cm3 | Boiling Point | 392.8ºC at 760 mmHg | |
| Molecular Formula | C12H16N2O | Melting Point | 62-64ºC | |
| MSDS | N/A | Flash Point | 191.3ºC | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
| Name | bufotenin |
|---|---|
| Synonym | More Synonyms |
| Density | 1.178g/cm3 |
|---|---|
| Boiling Point | 392.8ºC at 760 mmHg |
| Melting Point | 62-64ºC |
| Molecular Formula | C12H16N2O |
| Molecular Weight | 204.26800 |
| Flash Point | 191.3ºC |
| Exact Mass | 204.12600 |
| PSA | 39.26000 |
| LogP | 1.97760 |
| Vapour Pressure | 9.89E-07mmHg at 25°C |
| Index of Refraction | 1.646 |
| Storage condition | Refrigerator, Under Inert Atmosphere |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302 + H312 + H332-H319 |
| Precautionary Statements | P210-P280-P305 + P351 + P338 |
| Hazard Codes | F,Xn |
| Risk Phrases | 11-20/21/22-36 |
| Safety Phrases | 16-36/37 |
| RIDADR | UN 1544 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 3 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Psychedelic 5-methoxy-N,N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions.
Curr. Drug Metab. 11(8) , 659-66, (2010) 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) belongs to a group of naturally-occurring psychoactive indolealkylamine drugs. It acts as a nonselective serotonin (5-HT) agonist and causes many physiolog... |
|
|
Isolation of N,N-dimethyl and N-methylserotonin 5-O-β-glucosides from immature Zanthoxylum piperitum seeds.
Biosci. Biotechnol. Biochem. 74(9) , 1951-2, (2010) Two serotonin derivatives, N,N-dimethylserotonin 5-O-β-glucoside (1a) and N-methylserotonin 5-O-β-glucoside (1b) were isolated from immature seeds of Zanthoxylum piperitum. Their structures were deter... |
|
|
A critical review of reports of endogenous psychedelic N, N-dimethyltryptamines in humans: 1955-2010.
Drug Test. Anal. 4(7-8) , 617-35, (2012) Three indole alkaloids that possess differing degrees of psychotropic/psychedelic activity have been reported as endogenous substances in humans; N,N-dimethyltryptamine (DMT), 5-hydroxy-DMT (bufotenin... |
| Bufotenin |
| Cohoba |
| Mapine |
| Mappine |
| Mappin |
| Bufotenine |
| N,N-Dimethylserotonin |
| EINECS 207-667-9 |
| 3-[2-(dimethylamino)ethyl]-1H-indol-5-ol |
| Dimethylserotonin |
| 5-hydroxy-N,N-dimethyltryptamine |
| N,N-Dimethyl-5-hydroxytryptamine |
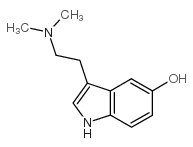
 CAS#:1019-45-0
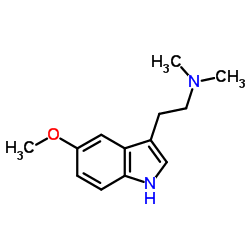
CAS#:1019-45-0 CAS#:24424-99-5
CAS#:24424-99-5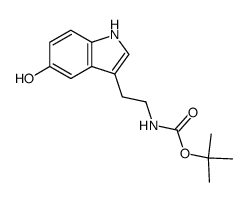 CAS#:53157-48-5
CAS#:53157-48-5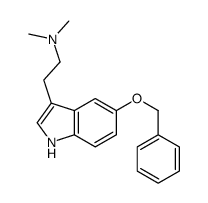 CAS#:25390-67-4
CAS#:25390-67-4 CAS#:161202-91-1
CAS#:161202-91-1 CAS#:64656-15-1
CAS#:64656-15-1 CAS#:66521-34-4
CAS#:66521-34-4 CAS#:61-50-7
CAS#:61-50-7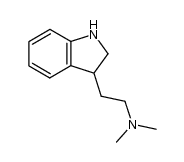 CAS#:38662-17-8
CAS#:38662-17-8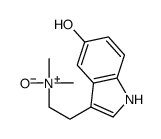 CAS#:1019-44-9
CAS#:1019-44-9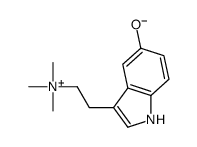 CAS#:487-91-2
CAS#:487-91-2 CAS#:5787-02-0
CAS#:5787-02-0