Ochratoxin B
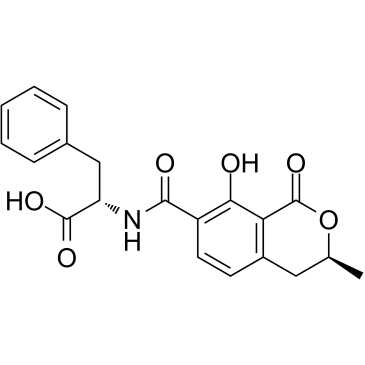
Ochratoxin B structure
|
Common Name | Ochratoxin B | ||
|---|---|---|---|---|
| CAS Number | 4825-86-9 | Molecular Weight | 369.368 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 632.4±55.0 °C at 760 mmHg | |
| Molecular Formula | C20H19NO6 | Melting Point | 221ºC | |
| MSDS | USA | Flash Point | 336.2±31.5 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
Use of Ochratoxin BOchratoxin B, a secondary metabolite of Aspergillus ochraceus, is the nonchlorinated analogue of the mycotoxin Ochratoxin A. Ochratoxin B has been shown to reduce the toxic effects of Ochratoxin A, and it is one of the most potent renal carcinogens in rodents[1][2]. |
| Name | (2S)-2-[[(3R)-8-hydroxy-3-methyl-1-oxo-3,4-dihydroisochromene-7-carbonyl]amino]-3-phenylpropanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Ochratoxin B, a secondary metabolite of Aspergillus ochraceus, is the nonchlorinated analogue of the mycotoxin Ochratoxin A. Ochratoxin B has been shown to reduce the toxic effects of Ochratoxin A, and it is one of the most potent renal carcinogens in rodents[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 632.4±55.0 °C at 760 mmHg |
| Melting Point | 221ºC |
| Molecular Formula | C20H19NO6 |
| Molecular Weight | 369.368 |
| Flash Point | 336.2±31.5 °C |
| Exact Mass | 369.121246 |
| PSA | 112.93000 |
| LogP | 3.06 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.623 |
| InChIKey | DAEYIVCTQUFNTM-ABAIWWIYSA-N |
| SMILES | CC1Cc2ccc(C(=O)NC(Cc3ccccc3)C(=O)O)c(O)c2C(=O)O1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302-H312-H319-H332 |
| Precautionary Statements | P210-P280-P305 + P351 + P338 |
| Hazard Codes | Xn,T,F |
| Risk Phrases | 22-36/37/38-65-48/23/24/25-36/38-11-46-45 |
| Safety Phrases | 26-36-45-16-53 |
| RIDADR | UN 3462 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | AY6825000 |
| Packaging Group | I |
| Hazard Class | 6.1(a) |
| HS Code | 39229000 |
|
~% 
Ochratoxin B CAS#:4825-86-9 |
| Literature: Gillman, Ivan G.; Yezek, Jennifer M.; Manderville, Richard A. Chemical Communications, 1998 , # 6 p. 647 - 648 |
|
~% 
Ochratoxin B CAS#:4825-86-9 |
| Literature: Brow, Mark E.; Dai, Jian; Park, Gyungse; Wright, Marcus W.; Gillman, Ivan G.; Manderville, Richard A. Photochemistry and Photobiology, 2002 , vol. 76, # 6 p. 649 - 656 |
|
~%
Detail
|
| Literature: Hadjeba-Medjdoub, Kheira; Tozlovanu, Mariana; Pfohl-Leszkowicz, Annie; Frenette, Christine; Paugh, Robert J.; Manderville, Richard A. Chemical Research in Toxicology, 2012 , vol. 25, # 1 p. 181 - 190 |
|
Wavelength-dependent degradation of ochratoxin and citrinin by light in vitro and in vivo and its implications on Penicillium.
Toxins (Basel.) 4(12) , 1535-51, (2012) It has previously been shown that the biosynthesis of the mycotoxins ochratoxin A and B and of citrinin by Penicillium is regulated by light. However, not only the biosynthesis of these mycotoxins, bu... |
|
|
Determination of ochratoxin A in wine by means of immunoaffinity column clean-up and high-performance liquid chromatography.
J. Chromatogr. A. 864(1) , 89-101, (1999) A new and accurate method to quantify ochratoxin A (OA) in table wine has been developed. The method uses commercial immunoaffinity columns for clean-up and high-performance liquid chromatography (HPL... |
|
|
Determination of ochratoxin A in grains by immuno-ultrafiltration and HPLC-fluorescence detection after postcolumn derivatisation in an electrochemical cell.
Anal. Bioanal. Chem 400(8) , 2615-22, (2011) The paper presents a new sample clean-up method based on immuno-ultrafiltration for the analysis of ochratoxin A in cereals. In contrast to immunoaffinity chromatography, in immuno-ultrafiltration, th... |
| OCHRATOXIN B |
| Ochratoxin B solution |
| ochratoxin A |
| N-{[(3R)-8-hydroxy-3-methyl-1-oxo-3,4-dihydro-1H-isochromen-7-yl]-carbonyl}-L-phenylalanine |
| N-{[(3R)-8-Hydroxy-3-methyl-1-oxo-3,4-dihydro-1H-isochromen-7-yl]carbonyl}-L-phenylalanine |
| Alanine,N-((8-hydroxy-3-methyl-1-oxo-7-isochromanyl)carbonyl)-3-phenyl-,(-) |
| N-{[(3R)-8-hydroxy-3-methyl-1-oxo-7-isochromanyl]carbonyl}-3-phenyl-L-alanine |
| (R)-N-[(3,4-Dihydro-8-hydroxy-3-methyl-1-oxo-1H-2-benzopyran-7-yl)carboxyl]phenylalanine |
| L-Phenylalanine, N-[[(3R)-3,4-dihydro-8-hydroxy-3-methyl-1-oxo-1H-2-benzopyran-7-yl]carbonyl]- |
| L-Phenylalanine,N-((3,4-dihydro-8-hydroxy-3-methyl-1-oxo-1H-2-benzopyran-7-yl)carbonyl)-,(R) |
| Ochratoxin B(OTB) |
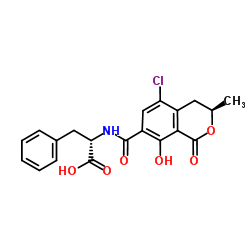
![N-{[(3R)-5,8-dihydroxy-3-methyl-1-oxo-3,4-dihydro-1H-isochromen-7-yl]carbonyl}-L-phenylalanine structure](https://image.chemsrc.com/caspic/161/205034-32-8.png)