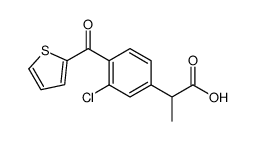
Suprofen
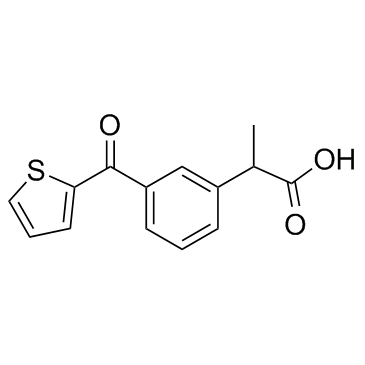
Suprofen structure
|
Common Name | Suprofen | ||
|---|---|---|---|---|
| CAS Number | 40828-46-4 | Molecular Weight | 260.308 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 442.6±30.0 °C at 760 mmHg | |
| Molecular Formula | C14H12O3S | Melting Point | 278ºC | |
| MSDS | Chinese USA | Flash Point | 221.5±24.6 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of SuprofenSuprofen is a non-steroidal anti-inflammatory drug (NSAID). Target: PGE synthaseSuprofen is an NSAID.Suprofen is an ibuprofen-type anti-inflammatory analgesic and antipyretic. It inhibits prostaglandin synthesis and has been proposed as an anti-arthritic. suprofen was clinically effective but the differential suppression of prostanoids favors 200mg which spares 6-keto PGF1a [1, 2]. |
| Name | suprofen |
|---|---|
| Synonym | More Synonyms |
| Description | Suprofen is a non-steroidal anti-inflammatory drug (NSAID). Target: PGE synthaseSuprofen is an NSAID.Suprofen is an ibuprofen-type anti-inflammatory analgesic and antipyretic. It inhibits prostaglandin synthesis and has been proposed as an anti-arthritic. suprofen was clinically effective but the differential suppression of prostanoids favors 200mg which spares 6-keto PGF1a [1, 2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 442.6±30.0 °C at 760 mmHg |
| Melting Point | 278ºC |
| Molecular Formula | C14H12O3S |
| Molecular Weight | 260.308 |
| Flash Point | 221.5±24.6 °C |
| Exact Mass | 260.050720 |
| PSA | 82.61000 |
| LogP | 2.42 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.613 |
| Storage condition | -20°C Freezer |
| Water Solubility | soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | Missing Phrase - N15.00950417 |
| RIDADR | UN 3249 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2934999090 |
|
~79% 
Suprofen CAS#:40828-46-4 |
| Literature: Maji, Arun; Rana, Sujoy; Akanksha; Maiti, Debabrata Angewandte Chemie - International Edition, 2014 , vol. 53, # 9 p. 2428 - 2432 Angew. Chem., 2014 , vol. 126, # 9 p. 2460 - 2464,5 |
|
~% 
Suprofen CAS#:40828-46-4 |
| Literature: Janssen Pharmaceutica N.V. Patent: US4035376 A1, 1977 ; |
|
~% 
Suprofen CAS#:40828-46-4 |
| Literature: Janssen Pharmaceutica N.V. Patent: US4035376 A1, 1977 ; |
|
~% 
Suprofen CAS#:40828-46-4 |
| Literature: Janssen Pharmaceutica N.V. Patent: US4035376 A1, 1977 ; |
|
~% 
Suprofen CAS#:40828-46-4
Detail
|
| Literature: Neumann, Helfried; Brennfuehrer, Anne; Beller, Matthias Advanced Synthesis and Catalysis, 2008 , vol. 350, # 14-15 p. 2437 - 2442 |
|
~% 
Suprofen CAS#:40828-46-4 |
| Literature: Johnson and Johnson Patent: US4185100 A1, 1980 ; |
|
~51% 
Suprofen CAS#:40828-46-4 |
| Literature: Neumann, Helfried; Brennfuehrer, Anne; Beller, Matthias Chemistry - A European Journal, 2008 , vol. 14, # 12 p. 3645 - 3652 |
|
~% 
Suprofen CAS#:40828-46-4 |
| Literature: Maji, Arun; Rana, Sujoy; Akanksha; Maiti, Debabrata Angewandte Chemie - International Edition, 2014 , vol. 53, # 9 p. 2428 - 2432 Angew. Chem., 2014 , vol. 126, # 9 p. 2460 - 2464,5 |
|
~% 
Suprofen CAS#:40828-46-4 |
| Literature: Camps, Pelayo; Gimenez, Silvia; Farres, Xavier; Mauleon, David; Carganico, Germano Liebigs Annalen der Chemie, 1993 , # 6 p. 641 - 644 |
| Precursor 9 | |
|---|---|
| DownStream 2 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Suprofen, a new peripheral analgesic.
J. Pharmacol. Exp. Ther. 214(1) , 16-23, (1980) The antinociceptive properties of suprofen [alpha-methyl-4-(thienylcarbonyl)benzene acetic acid] are described in a pathologically induced hyperalgesic model, the rat adjuvant arthritis flexion test. ... |
|
|
Suprofen: the pharmacology and clinical efficacy of a new non-narcotic peripheral analgesic.
Clin. Rheum. Dis. 10(2) , 353-68, (1984) Suprofen is a potent, peripherally-acting, non-narcotic analgesic agent. The mechanism of action of the compound involves inhibition of prostaglandin biosynthesis and, perhaps, direct antagonism of th... |
|
|
Suprofen, a potent antagonist of acetic acid-induced writhing in rats.
Arzneimittelforschung 25(10) , 1505-9, (1975) A new standardized acetic acid-induced writhing test in rats is described in detail and its methodology is discussed briefly. The described method has proved to be useful for evaluating the anti-writh... |
| EINECS 255-096-9 |
| p-(2-thenoyl)hydratropic acid |
| Supranol |
| Profenol |
| 2-[4-(2-Thienylcarbonyl)phenyl]propanoic acid |
| Suprol |
| Suprocil |
| Anemet |
| 2-[4-(thiophen-2-ylcarbonyl)phenyl]propanoic acid |
| Dolasteron |
| Profenal |
| Benzeneacetic acid, α-methyl-4-(2-thienylcarbonyl)- |
| Suprofen |
| Srendam |
| suprofenum [INN_la] |
| TN 762 |
| 2-(4-thenoyl)phenylpropanoic acid |
| maldocil |
| Topalgic |

![diethyl methyl[4-(2-thienylcarbonyl)phenyl]malonate structure](https://image.chemsrc.com/caspic/486/52779-57-4.png)
![2-[4-(thiophene-2-carbonyl)phenyl]propanenitrile structure](https://image.chemsrc.com/caspic/441/52779-85-8.png)


![1-[4-(2-Thienylcarbonyl)phenyl]-1-propanone structure](https://image.chemsrc.com/caspic/181/66952-47-4.png)

 CAS#:52779-81-4
CAS#:52779-81-4![sodium 2-[4-(2-thienylcarbonyl)phenyl]propionate structure](https://image.chemsrc.com/caspic/148/52779-97-2.png) CAS#:52779-97-2
CAS#:52779-97-2