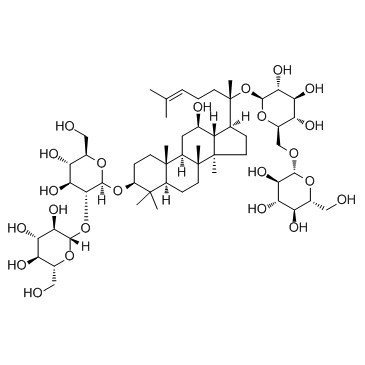
Ginsenoside Compound K
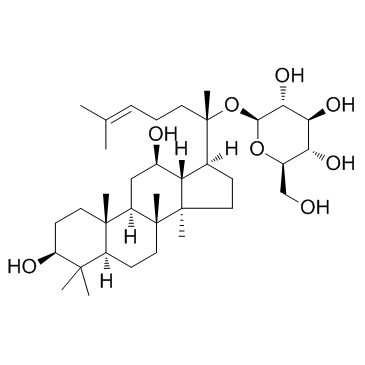
Ginsenoside Compound K structure
|
Common Name | Ginsenoside Compound K | ||
|---|---|---|---|---|
| CAS Number | 39262-14-1 | Molecular Weight | 622.873 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 723.1±60.0 °C at 760 mmHg | |
| Molecular Formula | C36H62O8 | Melting Point | 178 °C | |
| MSDS | USA | Flash Point | 391.1±32.9 °C | |
Use of Ginsenoside Compound KGinsenoside C-K, a bacterial metabolite of G-Rb1, exhibits anti-inflammatory effects by reducing iNOS and COX-2. Ginsenoside C-K exhibits an inhibition against the activity of CYP2C9 and CYP2A6 in human liver microsomes with IC50s of 32.0±3.6 μM and 63.6±4.2 μM, respectively. |
| Name | (2S,3R,4S,5S,6R)-2-[(2S)-2-[(3S,5R,8R,9R,10R,12R,13R,14R,17S)-3,12-dihydroxy-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]-6-methylhept-5-en-2-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|---|
| Synonym | More Synonyms |
| Description | Ginsenoside C-K, a bacterial metabolite of G-Rb1, exhibits anti-inflammatory effects by reducing iNOS and COX-2. Ginsenoside C-K exhibits an inhibition against the activity of CYP2C9 and CYP2A6 in human liver microsomes with IC50s of 32.0±3.6 μM and 63.6±4.2 μM, respectively. |
|---|---|
| Related Catalog | |
| Target |
COX-2 iNOS CYP2C9:32 μM (IC50) CYP2A6:63.6 μM (IC50) |
| In Vitro | Ginsenoside C-K, a bacterial metabolite of G-Rb1, exhibits anti-inflammatory effects mainly by reducing inducible nitric oxide synthase (iNOS), cyclooxygenase (COX)-2, and proinflammatory cytokines. Ginsenoside C-K suppresses the expression of proinflammatory cytokines by downregulating the activities of IRAK-1, MAPKs, IKK-α, and NF-κB in LPS-treated murine peritoneal macrophages. Ginsenoside C-K also suppresses the expression of iNOS and COX-2 by inhibiting NF-κB signaling in LPS-stimulated RAW264.7 cells. In zymosan-treated bone-marrow-derived macrophages (BMDMs) and RAW264.7 cells, Ginsenoside C-K inhibits inflammatory responses by negatively regulating the secretion of proinflammatory cytokines, the activation of MAPKs, and the generation of ROS. In addition, anti-inflammatory activity of Ginsenoside C-K has been observed in LPS-stimulated microglial cells. Ginsenoside C-K hinders inflammatory responses by controlling both the generation of ROS and the activities of MAPKs, NF-κB, and AP-1[1]. Ginsenoside C-K, a major metabolite of ginsenosides in the gastrointestinal tract, inhibits NF-κB signaling in a PXR-dependent manner. Ginsenoside C-K is shown to promote recovery of dextran sulfate sodium (DSS) -induced colitis by suppressing NF-κB activation. Ginsenoside C-K significantly reduces TNF-α-induced upregulation of IL-1β and iNOS mRNA levels, and restores the mRNA levels of PXR and CYP3A4 in LS174T cells[2]. Ginsenoside C-K, one of the intestinal metabolites of 20(S)-protopanaxadiol derivatives, exhibits an inhibition against the activity of CYP2C9 in human liver microsomes with an IC50 value of 32.0±3.6 μM, a weak inhibition against the activity of CYP2A6 in human liver microsomes with an IC50 value of 63.6±4.2 μM, and an even weaker inhibition against the activity of CYP2D6 in human liver microsomes with an IC50 value of more than 100 μM[4]. |
| In Vivo | The weight of the collagen-induced arthritis (CIA) mice increases slowly and is significantly less than that of the normal DBA/1 mice beginning on d 3 after injection of the emulsion. Ginsenoside C-K (28, 56, and 112 mg/kg) mice recover their weight by d 32 after the emulsion injection. Ginsenoside C-K (56 and 112 mg/kg) and Methotrexate (MTX)-treated (2 mg/kg) mice show significantly increased body weight on d 50 as compared with CIA mice. Hind paw-swelling began on d 24 post-immunization. CIA mice are treated from d 28 to d 50. Arthritis scores are measured every 4 d beginning on d 24. Ginsenoside C-K (56 and 112 mg/kg) significantly reduces the arthritis scores of the mice on d 51[3]. |
| Cell Assay | LS174T cells are seeded in cell imaging dish. After overnight incubation, cells are treated with ginseng saponin extract (GSE) (100 μg/mL), Rb1 (10 μM), or Ginsenoside C-K (10 μM) for 3 hours, followed by an additional incubation with or without TNF-α (20 ng/mL) for 6 hours. At the end of the incubation, cells are harvested and fixed with 4% paraformaldehyde solution at 20°C for 20 minutes. After washing in PBS, cells are permeabilized with 0.2% Triton X-100 in PBS at room temperature for 5 minutes. After incubation in blocking buffer containing 0.1% Triton X-100 and 5% bovine serum albumin, cells are incubated with rabbit NF-κB p65 antibody at 4°C overnight and then with Alexa Fluor 488-conjugated anti-rabbit IgG antibody at room temperature for 30 minutes in 1% bovine serum albumin in PBS. Fluorescence photographs are obtained using a Zeiss 710 confocal microscope[2]. |
| Animal Admin | Mice[3] Specific pathogen-free DBA/1 mice (male, 18±2 g) are used. DBA/1 mice are injected intradermally twice with 0.1 mL of this emulsion (containing 100 mg of chicken type II collagen (CII)/mouse) in the back and the base of the tail. The day of the first immunization is defined as d 0, and the booster injection is administered into the back on d 21. After the onset of arthritis, animals are randomly divided into five groups, and each experimental group consists of ten mice. Mice with CIA are intragastrically administered Ginsenoside C-K (28, 56, or 112 mg/kg) once per day or MTX (2 mg/kg) once every 3 d from d 28 to d 51 after immunization. Normal and CIA mice are administered an equal volume of vehicle (CMC-Na) at the same time[3]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 723.1±60.0 °C at 760 mmHg |
| Melting Point | 178 °C |
| Molecular Formula | C36H62O8 |
| Molecular Weight | 622.873 |
| Flash Point | 391.1±32.9 °C |
| Exact Mass | 622.444458 |
| PSA | 139.84000 |
| LogP | 5.50 |
| Vapour Pressure | 0.0±5.3 mmHg at 25°C |
| Index of Refraction | 1.572 |
| RIDADR | NONH for all modes of transport |
|---|
|
~83% 
Ginsenoside Com... CAS#:39262-14-1 |
| Literature: Akao, Teruaki; Kanaoka, Matao; Kobashi, Kyoichi Biological & Pharmaceutical Bulletin, 1998 , vol. 21, # 3 p. 245 - 249 |
|
~99% 
Ginsenoside Com... CAS#:39262-14-1 |
| Literature: Atopkina; Denisenko Chemistry of Natural Compounds, 2006 , vol. 42, # 4 p. 452 - 458 |
|
~% 
Ginsenoside Com... CAS#:39262-14-1 |
| Literature: Atopkina, Lyubov N.; Denisenko, Vladimir A.; Uvarova, Nina I.; Elyakov, Georgi B. Carbohydrate Research, 1988 , vol. 177, p. 101 - 110 |
|
~% 
Ginsenoside Com... CAS#:39262-14-1 |
| Literature: Atopkina, Lyubov N.; Denisenko, Vladimir A.; Uvarova, Nina I.; Elyakov, Georgi B. Carbohydrate Research, 1988 , vol. 177, p. 101 - 110 |
|
~% 
Ginsenoside Com... CAS#:39262-14-1 |
| Literature: Atopkina, Lyubov N.; Denisenko, Vladimir A.; Uvarova, Nina I.; Elyakov, Georgi B. Carbohydrate Research, 1988 , vol. 177, p. 101 - 110 |
| Compound K |
| X1141 |
| (3β,12β)-3,12-Dihydroxydammar-24-en-20-yl β-D-glucopyranoside |
| N1890 |
| β-D-Glucopyranoside, (3β,12β)-3,12-dihydroxydammar-24-en-20-yl |
| Ginsenoside K |
| Ginsenoside C-K |