indium(iii) isopropoxide
indium(iii) isopropoxide structure
|
Common Name | indium(iii) isopropoxide | ||
|---|---|---|---|---|
| CAS Number | 38218-24-5 | Molecular Weight | 292.07900 | |
| Density | 0.808 g/mL at 25ºC | Boiling Point | 82℃ | |
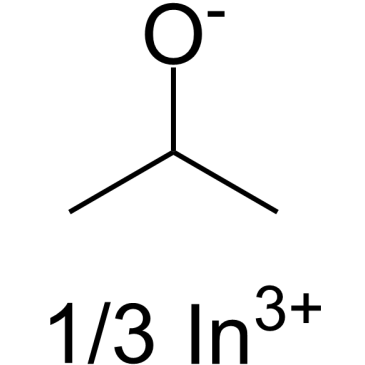
| Molecular Formula | C3H8O.1/3In | Melting Point | 145ºC (dec.) | |
| MSDS | N/A | Flash Point | 10ºC | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
Use of indium(iii) isopropoxideIndium(III) Isopropoxide is an organo-metallic compound. Indium(III) Isopropoxide uesd as a hydrogen transfer catalyst for conversion of benzylic alcohols into aldehydes or ketones via Oppenauer oxidation. Indium(III) Isopropoxide also can be used as metal precursor[1][2]. |
| Name | Indium tri(2-propanolate) |
|---|---|
| Synonym | More Synonyms |
| Description | Indium(III) Isopropoxide is an organo-metallic compound. Indium(III) Isopropoxide uesd as a hydrogen transfer catalyst for conversion of benzylic alcohols into aldehydes or ketones via Oppenauer oxidation. Indium(III) Isopropoxide also can be used as metal precursor[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Indium(III) isopropoxide as an Oppenaueroxidation catalyst, and the conversion of primary or secondary alcohols into the corresponding aldehydes or ketones was promoted at room temperature using pivalaldehyde as an oxidant[1]. |
| References |
| Density | 0.808 g/mL at 25ºC |
|---|---|
| Boiling Point | 82℃ |
| Melting Point | 145ºC (dec.) |
| Molecular Formula | C3H8O.1/3In |
| Molecular Weight | 292.07900 |
| Flash Point | 10ºC |
| Exact Mass | 292.05300 |
| PSA | 69.18000 |
| LogP | 2.47590 |
| Index of Refraction | n20/D1.383 |
| InChIKey | OVZUSPADPSOQQN-UHFFFAOYSA-N |
| SMILES | CC(C)[O-].CC(C)[O-].CC(C)[O-].[In+3] |
| Water Solubility | Soluble in alcohols, ketones and esters. |
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H319-H336 |
| Precautionary Statements | P210-P261-P305 + P351 + P338 |
| Hazard Codes | F: Flammable;Xi: Irritant; |
| Risk Phrases | 36/37/38-41-11 |
| Safety Phrases | 26-36/37/39-36-33-16 |
| RIDADR | UN 1219 3/PG 2 |
|
Ambient pressure syntheses of size-controlled corundum-type In2O3 nanocubes.
J. Am. Chem. Soc. 29th ed., 128 , 9326, (2006) Hydrolysis of In(O-iPr)3 by 10 molar excess of water at 90 degrees C in a surfactant/solvent mixture of oleylamine/oleic acid/trioctylamine provides very small nanoparticles (<5 nm in diameter) of In(... |
|
|
Caruntu, D.; et al.
J. Phys. Chem. C Nanomater. Interfaces 11th ed., 114 , 4875, (2010)
|
| MFCD00210318 |