2-(1h-indol-3-yl)ethanaminhydrochlorid
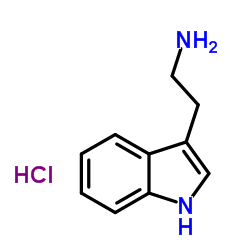
2-(1h-indol-3-yl)ethanaminhydrochlorid structure
|
Common Name | 2-(1h-indol-3-yl)ethanaminhydrochlorid | ||
|---|---|---|---|---|
| CAS Number | 343-94-2 | Molecular Weight | 196.677 | |
| Density | 1.157g/cm3 | Boiling Point | 378.8ºC at 760mmHg | |
| Molecular Formula | C10H13ClN2 | Melting Point | 253-255 °C(lit.) | |
| MSDS | USA | Flash Point | 187.7ºC | |
Use of 2-(1h-indol-3-yl)ethanaminhydrochloridTryptamine (hydrochloride) is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 2-(1H-indol-3-yl)ethanamine,hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Tryptamine (hydrochloride) is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.157g/cm3 |
|---|---|
| Boiling Point | 378.8ºC at 760mmHg |
| Melting Point | 253-255 °C(lit.) |
| Molecular Formula | C10H13ClN2 |
| Molecular Weight | 196.677 |
| Flash Point | 187.7ºC |
| Exact Mass | 196.076721 |
| PSA | 41.81000 |
| LogP | 3.17140 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | NL4375000 |
| HS Code | 2942000000 |
|
~78% 
2-(1h-indol-3-y... CAS#:343-94-2 |
| Literature: Organic Letters, , vol. 6, # 22 p. 3885 - 3888 |
|
~92% 
2-(1h-indol-3-y... CAS#:343-94-2 |
| Literature: Synthetic Communications, , vol. 34, # 7 p. 1317 - 1323 |
|
~87% 
2-(1h-indol-3-y... CAS#:343-94-2 |
| Literature: Chemistry of Natural Compounds, , vol. 21, # 4 p. 502 - 509 Khimiya Prirodnykh Soedinenii, , vol. 21, # 4 p. 536 - 544 |
|
~83% 
2-(1h-indol-3-y... CAS#:343-94-2 |
| Literature: Chemistry of Natural Compounds, , vol. 17, p. 149 - 152 Khimiya Prirodnykh Soedinenii, , vol. 17, # 2 p. 192 - 195 |
|
~% 
2-(1h-indol-3-y... CAS#:343-94-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 37, # 25 p. 4307 - 4316 |
|
~% 
2-(1h-indol-3-y... CAS#:343-94-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 37, # 25 p. 4307 - 4316 |
|
~% 
2-(1h-indol-3-y... CAS#:343-94-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 37, # 25 p. 4307 - 4316 |
|
~% 
2-(1h-indol-3-y... CAS#:343-94-2 |
| Literature: Chemistry of Natural Compounds, , vol. 17, p. 149 - 152 Khimiya Prirodnykh Soedinenii, , vol. 17, # 2 p. 192 - 195 |
|
~% 
2-(1h-indol-3-y... CAS#:343-94-2 |
| Literature: Chemistry of Natural Compounds, , vol. 17, p. 149 - 152 Khimiya Prirodnykh Soedinenii, , vol. 17, # 2 p. 192 - 195 |
| Precursor 10 | |
|---|---|
| DownStream 9 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
|
|
New procedure of selected biogenic amines determination in wine samples by HPLC.
Anal. Chim. Acta 834 , 58-66, (2014) A new procedure for determination of biogenic amines (BA): histamine, phenethylamine, tyramine and tryptamine, based on the derivatization reaction with 2-chloro-1,3-dinitro-5-(trifluoromethyl)-benzen... |
|
|
Enantioselective formal synthesis of ent-rhynchophylline and ent-isorhynchophylline.
Chem. Commun. (Camb.) 49(19) , 1954-6, (2013) Starting from (S)-tryptophanol, a formal synthesis of ent-rhynchophylline and ent-isorhynchophylline, involving stereoselective cyclocondensation, spirocyclization, and alkylation reactions, and the f... |
| triptamine hydrochloride |
| 3-(2-Aminoethyl)indole Hydrochloride |
| Indole, 3- (2-aminoethyl)-, monohydrochloride |
| serotonin hydrochloride |
| TryptaMine Hydrochloride |
| Indole-3-ethylamine hydrochloride |
| 2-(1H-Indol-3-yl)éthanamine chlorhydrate |
| 2-(1H-Indol-3-yl)ethanamine hydrochloride |
| MFCD00012682 |
| 1H-Indole-3-ethanamine, hydrochloride (1:1) |
| EINECS 206-446-4 |
| tryptamine HCl |
| 2-(1h-indol-3-yl)ethanaminhydrochlorid |
| 1H-Indole-3-ethanamine,monohydrochloride |
| 2-(1H-Indol-3-yl)ethanamine hydrochloride (1:1) |
| Tryptamine monohydrochloride |
| 3-(2-Aminoethyl)-1H-indole monohydrochloride |

![1H-Pyrido[3,4-b]indol-1-one,2,3,4,9-tetrahydro- structure](https://image.chemsrc.com/caspic/106/17952-82-8.png)
 CAS#:50-67-9
CAS#:50-67-9 CAS#:443-31-2
CAS#:443-31-2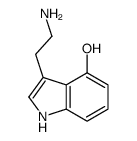 CAS#:570-14-9
CAS#:570-14-9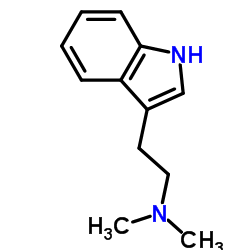 CAS#:61-50-7
CAS#:61-50-7![1-phenyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole structure](https://image.chemsrc.com/caspic/484/3790-45-2.png) CAS#:3790-45-2
CAS#:3790-45-2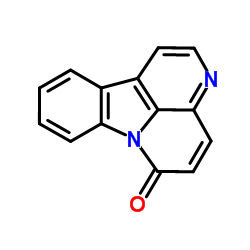 CAS#:479-43-6
CAS#:479-43-6![{Pyrrolo[2,1:2,3]pyrimido[1,6:1,2]pyrido[3,} 4-b]indole, 1,2,3,3a,5,6,11,1b,12,13-decahydro-, cis- structure](https://image.chemsrc.com/caspic/036/20069-07-2.png) CAS#:20069-07-2
CAS#:20069-07-2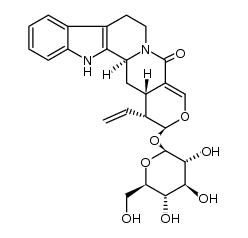 CAS#:22621-94-9
CAS#:22621-94-9![(4S)-4β-[[(1R)-1,2,3,4-Tetrahydro-β-carboline-1β-yl]methyl]-5β-ethenyl-6α-(β-D-glucopyranosyloxy)-5,6-dihydro-4H-pyran-3-carboxylic acid methyl ester structure](https://image.chemsrc.com/caspic/220/19456-89-4.png) CAS#:19456-89-4
CAS#:19456-89-4
