n-tert-butyl-n-[(e)-phenylmethylene]amine oxide
![n-tert-butyl-n-[(e)-phenylmethylene]amine oxide Structure](https://image.chemsrc.com/caspic/242/3376-24-7.png)
n-tert-butyl-n-[(e)-phenylmethylene]amine oxide structure
|
Common Name | n-tert-butyl-n-[(e)-phenylmethylene]amine oxide | ||
|---|---|---|---|---|
| CAS Number | 3376-24-7 | Molecular Weight | 177.243 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 283.3±23.0 °C at 760 mmHg | |
| Molecular Formula | C11H15NO | Melting Point | 71-75ºC | |
| MSDS | Chinese USA | Flash Point | 118.5±15.4 °C | |
Use of n-tert-butyl-n-[(e)-phenylmethylene]amine oxideN-tert-Butyl-α-phenylnitrone is a nitrone-based free radical scavenger that forms nitroxide spin adducts. N-tert-Butyl-α-phenylnitrone inhibits COX2 catalytic activity. N-tert-Butyl-α-phenylnitrone has potent ROS scavenging, anti-inflammatory, neuroprotective, anti-aging and anti-diabetic activities, and can penetrate the blood-brain barrier[1][2][3][4]. |
| Name | N-TERT-BUTYL-α-PHENYLNITRONE |
|---|---|
| Synonym | More Synonyms |
| Description | N-tert-Butyl-α-phenylnitrone is a nitrone-based free radical scavenger that forms nitroxide spin adducts. N-tert-Butyl-α-phenylnitrone inhibits COX2 catalytic activity. N-tert-Butyl-α-phenylnitrone has potent ROS scavenging, anti-inflammatory, neuroprotective, anti-aging and anti-diabetic activities, and can penetrate the blood-brain barrier[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
COX-2 Reactive oxygen species (ROS) |
| In Vitro | N-tert-Butyl-α-phenylnitrone (PBN) (25-100 µM) treatment leads to a significant decrease in 2,2'-azobis (2-amidinopropane) dihydrochloride (AAPH)-induced intracellular ROS accumulation. N-tert-Butyl-α-phenylnitrone also attenuates AAPH-induced cytotoxicity, matrix degradation, and apoptosis. N-tert-Butyl-α-phenylnitrone suppresses AAPH-induced activation of ERK/MAPK pathway. N-tert-Butyl-α-phenylnitrone has the potenial for intervertebral disc degeneration (IDD) research[1]. |
| In Vivo | N-tert-Butyl-α-phenylnitrone (PBN; 100 mg/kg; intraperitoneal injection; twice a day; C57Bl/6 mice) treatment not only abolishes the LPS-induced lipid peroxidation, nitrotyrosine residue levels, and GSH depletion, but also decreases the incidence of external malformations[2]. Animal Model: C57Bl/6 mice induced by lipopolysaccharide (LPS)[2] Dosage: 100 mg/kg Administration: Intraperitoneal injection; twice a day (on gestational day 8) Result: Abolished LPS-induced lipid peroxidation, nitrotyrosine residues, and GSH depletion. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 283.3±23.0 °C at 760 mmHg |
| Melting Point | 71-75ºC |
| Molecular Formula | C11H15NO |
| Molecular Weight | 177.243 |
| Flash Point | 118.5±15.4 °C |
| Exact Mass | 177.115356 |
| PSA | 28.75000 |
| LogP | 1.25 |
| Appearance of Characters | powder | white |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.552 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: soluble |
| Precursor 10 | |
|---|---|
| DownStream 8 | |
| HS Code | 2925290090 |
|---|---|
| Summary | 2925290090 other imines and their derivatives; salts thereof。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
The effect of normoxia and hypoxia on a prostate (PC-3) CD44/CD41 cell side fraction.
Anticancer Res. 31(2) , 487-94, (2011) It has been reported that human prostate-derived PC-3 cells that are CD44- and CD 41 (a2 B1)-positive are enriched in cancer stem cells. This study compared the effect of PC-3 cell proliferation under... |
|
|
Hydroxychloroquine-inhibited dengue virus is associated with host defense machinery.
J. Interferon Cytokine Res. 35(3) , 143-56, (2015) Hydroxychloroquine (HCQ) is an antimalarial drug also used in treating autoimmune diseases. Its antiviral activity was demonstrated in restricting HIV infection in vitro; however, the clinical implica... |
|
|
Systematic and Molecular Basis of the Antibacterial Action of Quinoxaline 1,4-Di-N-Oxides against Escherichia coli.
PLoS ONE 10 , e0136450, (2015) Quinoxaline 1,4-di-N-oxides (QdNOs) are widely known as potent antibacterial agents, but their antibacterial mechanisms are incompletely understood. In this study, the transcriptomic and proteomic pro... |
| N-tert-Butyl-alpha-phenylnitrone |
| phenyl t-butyl nitrone |
| N-Benzylidene-tert-butylamine N-oxide,PBN,Phenyl N-t-butylnitrone |
| N-tert-Butyl-α-phenylnitrone |
| Styryl phenyl sulfone |
| 2-Phenylethenyl phenyl sulfone |
| MFCD00008799 |
| EINECS 222-168-6 |
| N-Benzylidene-N-(2-methyl-2-propanyl)amine oxide |
| N-benzylidene-tert-butylamine N-oxide |
| n-tert-butyl-n-[(e)-phenylmethylene]amine oxide |
| N-benzylidene-t-butylamine N-oxide |
| PHENYL TRANS-STYRYL SULFONE 99 |
| Azane, (1,1-dimethylethyl)(phenylmethylene)-, oxide |
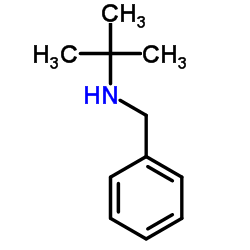 CAS#:3378-72-1
CAS#:3378-72-1 CAS#:100-52-7
CAS#:100-52-7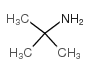 CAS#:75-64-9
CAS#:75-64-9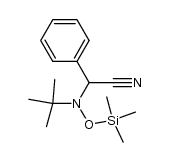 CAS#:105488-71-9
CAS#:105488-71-9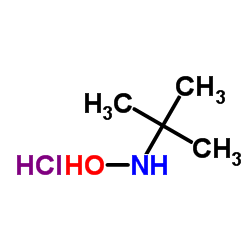 CAS#:57497-39-9
CAS#:57497-39-9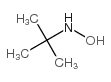 CAS#:16649-50-6
CAS#:16649-50-6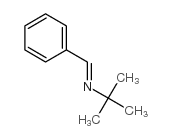 CAS#:6852-58-0
CAS#:6852-58-0 CAS#:3585-81-7
CAS#:3585-81-7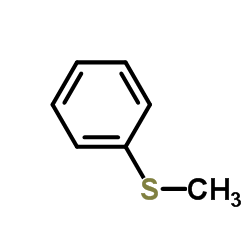 CAS#:100-68-5
CAS#:100-68-5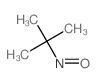 CAS#:917-95-3
CAS#:917-95-3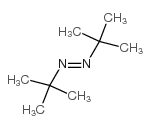 CAS#:927-83-3
CAS#:927-83-3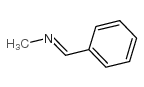 CAS#:622-29-7
CAS#:622-29-7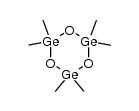 CAS#:16090-53-2
CAS#:16090-53-2 CAS#:1801-42-9
CAS#:1801-42-9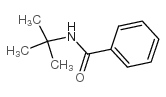 CAS#:5894-65-5
CAS#:5894-65-5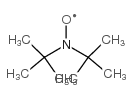 CAS#:2406-25-9
CAS#:2406-25-9 CAS#:35822-90-3
CAS#:35822-90-3
