Bisdemethoxycurcumin
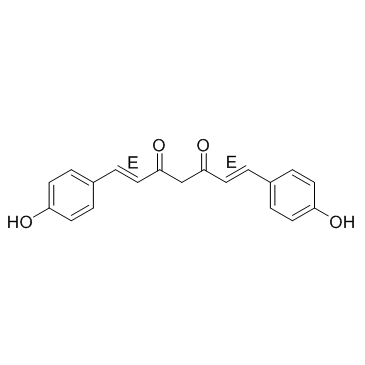
Bisdemethoxycurcumin structure
|
Common Name | Bisdemethoxycurcumin | ||
|---|---|---|---|---|
| CAS Number | 33171-05-0 | Molecular Weight | 308.328 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 551.3±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H16O4 | Melting Point | 221-223ºC | |
| MSDS | Chinese USA | Flash Point | 301.3±25.2 °C | |
Use of BisdemethoxycurcuminBisdemethoxycurcumin(Curcumin III; Didemethoxycurcumin) is a natural derivative of curcumin with anti-inflammatory and anti-cancer activities.IC50 value:Target: Anticancer natural compoundin vitro: BDMC-induced apoptosis was mediated by a combinatory inhibition of cytoprotective proteins, such as Bcl2 and heme oxygenase-1 and increased generation of reactive oxygen species. Intriguingly, BDMC-induced apoptosis was reversed with co-treatment of sr144528, a cannabinoid receptor (CBR) 2 antagonist, which was confirmed with genetic downregulation of the receptor using siCBR2 [1]. Induction of cell cycle arrest in HepG2 cells by NB and BDCur in combination was evidenced by accumulation of the G2/M cell population. Further investigation on the molecular mechanism showed that NB and BDCur in combination resulted in a significant decrease in the expression level of Cdc2 and cyclin B [2]. BDMC treatment activated Sirt1/AMPK signaling pathway. Moreover, downregulating Sirt1 by the pharmacological inhibitor nicotianamine or small interfering RNA blocked BDMC-mediated protection against t-BHP-mediated decrease in proliferation [4].in vivo: human gastric adenocarcinoma xenograft model was generated in vivo using nude mice and BDMC was observed to suppress the growth and activity of tumors, in addition to improving the physical and mental capacity of the mice [3]. |
| Name | Bisdemethoxycurcumin |
|---|---|
| Synonym | More Synonyms |
| Description | Bisdemethoxycurcumin(Curcumin III; Didemethoxycurcumin) is a natural derivative of curcumin with anti-inflammatory and anti-cancer activities.IC50 value:Target: Anticancer natural compoundin vitro: BDMC-induced apoptosis was mediated by a combinatory inhibition of cytoprotective proteins, such as Bcl2 and heme oxygenase-1 and increased generation of reactive oxygen species. Intriguingly, BDMC-induced apoptosis was reversed with co-treatment of sr144528, a cannabinoid receptor (CBR) 2 antagonist, which was confirmed with genetic downregulation of the receptor using siCBR2 [1]. Induction of cell cycle arrest in HepG2 cells by NB and BDCur in combination was evidenced by accumulation of the G2/M cell population. Further investigation on the molecular mechanism showed that NB and BDCur in combination resulted in a significant decrease in the expression level of Cdc2 and cyclin B [2]. BDMC treatment activated Sirt1/AMPK signaling pathway. Moreover, downregulating Sirt1 by the pharmacological inhibitor nicotianamine or small interfering RNA blocked BDMC-mediated protection against t-BHP-mediated decrease in proliferation [4].in vivo: human gastric adenocarcinoma xenograft model was generated in vivo using nude mice and BDMC was observed to suppress the growth and activity of tumors, in addition to improving the physical and mental capacity of the mice [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 551.3±45.0 °C at 760 mmHg |
| Melting Point | 221-223ºC |
| Molecular Formula | C19H16O4 |
| Molecular Weight | 308.328 |
| Flash Point | 301.3±25.2 °C |
| Exact Mass | 308.104858 |
| PSA | 74.60000 |
| LogP | 3.39 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.680 |
| Storage condition | -20°C |
| RIDADR | NONH for all modes of transport |
|---|
|
An efficient and economical MTT assay for determining the antioxidant activity of plant natural product extracts and pure compounds.
J. Nat. Prod. 73 , 1193-5, (2010) Antioxidants scavenge free radicals, singlet oxygen, and electrons in cellular redox reactions. The yellow MTT [3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide] is reduced to a purple fo... |
|
|
Molecularly imprinted polymers for cleanup and selective extraction of curcuminoids in medicinal herbal extracts.
Anal. Bioanal. Chem 407(3) , 803-12, (2015) This paper describes the synthesis of novel molecularly imprinted polymers (MIPs), prepared by a noncovalent imprinting approach, for cleanup and preconcentration of curcumin (CUR) and bisdemethoxycur... |
|
|
Isoxazole analogs of curcuminoids with highly potent multidrug-resistant antimycobacterial activity.
Eur. J. Med. Chem. 45 , 4446-57, (2010) Curcumin (1), demethoxycurcumin (2) and bisdemethoxycurcumin (3), the curcuminoid constituents of the medicinal plant Curcuma longa L., have been structurally modified to 55 analogs and antimycobacter... |
| 1,6-Heptadiene-3,5-dione, 1,7-bis(4-hydroxyphenyl)-, (E,E)- |
| bis(4-hydroxycinnamoyl)methane |
| Bis(p-hydroxycinnamoyl)methane |
| Bisdemethoxycurcumin |
| (1E,6E)-1,7-Bis(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione |
| (1E,6E)-1,7-bis(4-hydroxyphenyl)hepta-1,6-diene-3,5-dione |
| 1,6-Heptadiene-3,5-dione, 1,7-bis(4-hydroxyphenyl)-, (1E,6E)- |

